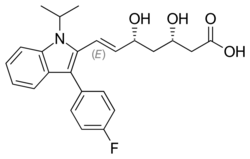
Chemistry:Fluvastatin
 | |
| Clinical data | |
|---|---|
| Trade names | Lescol, others |
| AHFS/Drugs.com | Monograph |
| MedlinePlus | a694010 |
| Pregnancy category |
|
| Routes of administration | By mouth (capsules, tablets) |
| ATC code | |
| Legal status | |
| Legal status | |
| Pharmacokinetic data | |
| Bioavailability | 24–30%[1][2] |
| Protein binding | >98%[2] |
| Metabolism | Hepatic: CYP2C9 (75%), CYP3A4 (20%), CYP2C8 (5%)[2][3] |
| Elimination half-life | 1–3 hours (capsule), 9 hours (XR formulations)[2][3] |
| Excretion | Faeces (95%), urine (5%)[2] |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| IUPHAR/BPS | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEBI | |
| ChEMBL | |
| Chemical and physical data | |
| Formula | C24H26FNO4 |
| Molar mass | 411.473 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| (verify) | |
Fluvastatin is a member of the statin drug class, used to treat hypercholesterolemia and to prevent cardiovascular disease.
It was patented in 1982 and approved for medical use in 1994.[4] It is on the World Health Organization's List of Essential Medicines.[5]
Adverse effects
Adverse effects are comparable to other statins. Common are nausea, indigestion, insomnia and headache. Myalgia (muscle pain), and rarely rhabdomyolysis, characteristic side effects for statins, can also occur.[6]
Interactions
Contrary to lovastatin, simvastatin and atorvastatin, fluvastatin has no relevant interactions with drugs that inhibit the liver enzyme CYP3A4, and a generally lower potential for interactions than most other statins. Fluconazole, a potent inhibitor of CYP2C9, does increase fluvastatin levels.[6]
Pharmacology
Mechanism of action
Fluvastatin works by blocking the liver enzyme HMG-CoA reductase, which facilitates an important step in cholesterol synthesis.[1]
Pharmacodynamics
In a Cochrane systematic review the dose-related magnitudes of fluvastatin on blood lipids was determined. Over the dose range of 10 to 80 mg/day total cholesterol was reduced by 10.7% to 24.9%, LDL cholesterol by 15.2% to 34.9%, and triglycerides by 3% to 17.5%.[7]
Pharmacokinetics
The drug is quickly and almost completely (98%) absorbed from the gut. Food intake slows down absorption, but does not decrease it. Due to its first-pass effect, bioavailability is lower: about 24–30%[2][1] according to different sources. Over 98% of the substance is bound to plasma proteins.[1]
Several cytochrome P450 enzymes (mainly CYP2C9, but also CYP3A4 and CYP2C8)[8] are involved in the metabolism of fluvastatin, which makes is less liable to interactions than most other statins. The main metabolite is inactive and is called "N-desisopropyl propionic acid" in the literature.[1][6]
93–95% of the drug is excreted via the feces, less than 2% of which in form of the original substance.[1]
Names
Fluvastatin is the INN.[9] Brandnames include Lescol, Canef, Vastin.
Research
Data from the Cholesterol Treatment Trialists’ (CTT) publication[10] was used to determine the effects of fluvastatin, atorvastatin and rosuvastatin on LDL cholesterol lowering and reduction of myocardial infarction. In two RCTs an average dose of 72 mg/day fluvastatin reduced LDL cholesterol by 31.9%, and reduced myocardial infarction, relative risk, 0.68 (95% CI 0.55 to 0.85) as compared to placebo. In five RCTs a mean atorvastatin dose of 26 mg/day reduced LDL cholesterol by 44.0% and reduced myocardial infarction, relative risk, 0.67 (95% CI 0.58 to 0.77) as compared to placebo. In four RCTs a mean rosuvastatin dose of 16 mg/day reduced LDL cholesterol by 48.8% and reduced myocardial infarction, relative risk, 0.82 (95% CI 0.73 to 0.93) as compared to placebo. Thus despite reducing LDL cholesterol by a much lesser amount with fluvastatin than atorvastatin and rosuvastatin, fluvastatin reduced myocardial infarction similarly to atorvastatin and to a greater degree than rosuvastatin.[7]
References
- ↑ 1.0 1.1 1.2 1.3 1.4 1.5 (in German) Austria-Codex. Vienna: Österreichischer Apothekerverlag. 2015.
- ↑ 2.0 2.1 2.2 2.3 2.4 2.5 "Pharmacokinetic comparison of the potential over-the-counter statins simvastatin, lovastatin, fluvastatin and pravastatin". Clinical Pharmacokinetics 47 (7): 463–74. 2008. doi:10.2165/00003088-200847070-00003. PMID 18563955.
- ↑ 3.0 3.1 "Lescol, Lescol XR (fluvastatin) dosing, indications, interactions, adverse effects, and more". Medscape Reference. WebMD. http://reference.medscape.com/drug/lescol-xl-fluvastatin-342456#showall.
- ↑ (in en) Analogue-based Drug Discovery. John Wiley & Sons. 2006. p. 472. ISBN 9783527607495. https://books.google.com/books?id=FjKfqkaKkAAC&pg=PA472.
- ↑ World Health Organization model list of essential medicines: 22nd list (2021). Geneva: World Health Organization. 2021. WHO/MHP/HPS/EML/2021.02.
- ↑ 6.0 6.1 6.2 (in German) Arzneistoff-Profile. 2 (26 ed.). Eschborn, Germany: Govi Pharmazeutischer Verlag. 2012. ISBN 978-3-7741-9846-3.
- ↑ 7.0 7.1 "Fluvastatin for lowering lipids". The Cochrane Database of Systematic Reviews (John Wiley & Sons, Ltd) 2018 (3): CD012282. March 2018. doi:10.1002/14651858.cd012282.pub2. PMID 29508377.
- ↑ Lescol Monograph on Drugs.com.
- ↑ "International Nonproprietary Names for Pharmaceutical Substances (INN). Recommended International Nonproprietary Names (Rec. INN): List 30". World Health Organization. 1990. https://www.who.int/medicines/publications/druginformation/innlists/RL30.pdf.
- ↑ "Efficacy and safety of cholesterol-lowering treatment: prospective meta-analysis of data from 90,056 participants in 14 randomised trials of statins". Lancet 366 (9493): 1267–78. October 2005. doi:10.1016/s0140-6736(05)67394-1. PMID 16214597.
 |
