Chemistry:Indazole

| |||
|
| |||
| Names | |||
|---|---|---|---|
| Preferred IUPAC name
1H-Indazole[1] | |||
| Identifiers | |||
3D model (JSmol)
|
|||
| ChEBI | |||
| ChEMBL | |||
| ChemSpider | |||
PubChem CID
|
|||
| UNII | |||
| |||
| |||
| Properties | |||
| C7H6N2 | |||
| Molar mass | 118.14 g/mol | ||
| Melting point | 147 to 149 °C (297 to 300 °F; 420 to 422 K) | ||
| Boiling point | 270 °C (518 °F; 543 K) | ||
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |||
| Infobox references | |||
Indazole, also called isoindazole, is a heterocyclic aromatic organic compound. This bicyclic compound consists of the fusion of benzene and pyrazole.
Indazole is an amphoteric molecule which can be protonated to an indazolium cation or deprotonated to an indazolate anion. The corresponding pKa values are 1.04 for the equilibrium between indazolium cation and indazole and 13.86 for the equilibrium between indazole and indazolate anion.[2]
Indazole derivatives display a broad variety of biological activities.
Indazoles are rare in nature. The alkaloids nigellicine, nigeglanine, and nigellidine are indazoles. Nigellicine was isolated from the widely distributed plant Nigella sativa L. (black cumin). Nigeglanine was isolated from extracts of Nigella glandulifera.
The Davis–Beirut reaction can generate 2H-indazoles.
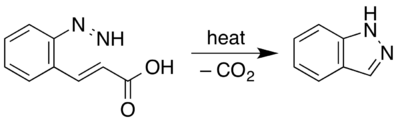
Indazole, C7H6N2, was obtained by E. Fischer (Ann. 1883, 221, p. 280) by heating ortho-hydrazine cinnamic acid,[3]
Some derivatives
- indazole-3-carboxylic acid
- Having a carboxylic acid group on carbon 3. Can be further modified to lonidamine.
See also
- Indole, an analog with only one nitrogen atom in position 1.
- Benzimidazole, an analog with the nitrogen atoms in positions 1 and 3.
- Simple aromatic rings
- 7-Nitroindazole, an indazole-based nitric oxide synthase inhibitor
References
- ↑ International Union of Pure and Applied Chemistry (2014). Nomenclature of Organic Chemistry: IUPAC Recommendations and Preferred Names 2013. The Royal Society of Chemistry. pp. 213. doi:10.1039/9781849733069. ISBN 978-0-85404-182-4.
- ↑ Catalan, Javier; Elguero, Jose (1987). "Basicity and Acidity of Azoles". Advances in Heterocyclic Chemistry Volume 41. 41. Elsevier. pp. 187–274. doi:10.1016/s0065-2725(08)60162-2. ISBN 9780120206414.
- ↑ Chisholm, Hugh, ed (1911). "Indazoles". Encyclopædia Britannica. 14 (11th ed.). Cambridge University Press. p. 371.
- Synthesis: Stadlbauer, W. (2002). "Product Class 2: 1H- and 2H-Indazoles". Category 2, Hetarenes and Related Ring Systems. Science of Synthesis. Houben-Weyl. doi:10.1055/sos-SD-012-00277. ISBN 9783131122711.
- Review: Schmidt, Andreas; Beutler, Ariane; Snovydovych, Bohdan (2008). "Recent Advances in the Chemistry of Indazoles". European Journal of Organic Chemistry 2008 (24): 4073–4095. doi:10.1002/ejoc.200800227.
 |