Chemistry:S-Nitrosoglutathione
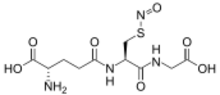
| |
| Names | |
|---|---|
| IUPAC name
L-γ-Glutamyl-S-nitroso-L-cysteinylglycine
| |
| Systematic IUPAC name
(2S)-2-Amino-5-({(2R)-1-[(carboxymethyl)amino]-3-(nitrososulfanyl)-1-oxopropan-2-yl}amino)-5-oxopentanoic acid | |
| Other names
Glutathione thionitrite; S-Nitroso-L-glutathione; SNOG; GSNO
| |
| Identifiers | |
3D model (JSmol)
|
|
| 3566211 | |
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| MeSH | S-Nitrosoglutathione |
PubChem CID
|
|
| RTECS number |
|
| UNII | |
| |
| |
| Properties | |
| C10H16N4O7S | |
| Molar mass | 336.32 g·mol−1 |
| log P | −2.116 |
| Acidity (pKa) | 2.212 |
| Basicity (pKb) | 11.785 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
S-Nitrosoglutathione (GSNO) is an endogenous S-nitrosothiol (SNO) that plays a critical role in nitric oxide (NO) signaling and is a source of bioavailable NO. NO coexists in cells with SNOs that serve as endogenous NO carriers and donors. SNOs spontaneously release NO at different rates and can be powerful terminators of free radical chain propagation reactions, by reacting directly with ROO• radicals, yielding nitro derivatives as end products.[1] NO is generated intracellularly by the nitric oxide synthase (NOS) family of enzymes: nNOS, eNOS and iNOS while the in vivo source of many of the SNOs is unknown. In oxygenated buffers, however, formation of SNOs is due to oxidation of NO to dinitrogen trioxide (N2O3).[2] Some evidence suggests that both exogenous NO and endogenously derived NO from nitric oxide synthases can react with glutathione to form GSNO.
GSNOR
The enzyme GSNO reductase (GSNOR) reduces S-nitrosoglutathione (GSNO) to an unstable intermediate, S-hydroxylaminoglutathione, which then rearranges to form glutathione sulfonamide, or in the presence of GSH, forms oxidized glutathione (GSSG) and hydroxylamine.[3][4][5] Through this catabolic process, GSNOR regulates the cellular concentrations of GSNO and plays a central role in regulating the levels of endogenous S-nitrosothiols and controlling protein S-nitrosylation-based signaling.

The generation of GSNO can serve as a stable and mobile NO pool which can effectively transduce NO signaling.[6][7] Unlike other low molecular weight messengers that bind to and activate target cellular receptors, NO signaling is mediated by a coordinating complex between NO and transition metals or target cellular proteins, often via S-nitrosylation of cysteine residues.[8][9][10] Studies suggest that NO metabolism has a significant role in human cardiovascular and respiratory diseases as well as in immune tolerance during organ transplantation.[11][12][13][14]
GSNO in Health and Disease
GSNO and NO concentrations regulate respiratory function by modulating airway tone and pro- and anti-inflammatory responses in the respiratory tract.[14][15] Because NO is a labile gas and endogenous levels are difficult to manipulate, it has been proposed that exogenous GSNO could be used to regulate circulating levels of NO and NO-derived species, and GSNO could have value in patients with pulmonary diseases such as cystic fibrosis. Consistent with this therapeutic goal, a recent study showed that acute treatment with aerosolized GSNO was well tolerated by cystic fibrosis patients.[14]
SNOs in the hepatic mitochondria appear to influence proper functioning of the liver. Mitochondrial SNO-proteins inhibit Complex I of the electron transport chain; modulate mitochondrial reactive oxygen species (ROS) production; influence calcium-dependent opening of the mitochondrial permeability transition pore; promote selective importation of mitochondrial proteins; and stimulate mitochondrial fission. Altered redox balance plays a crucial role in the pathogenesis of liver diseases including steatosis, steatohepatitis, and fibrosis. The ease of reversibility and the interplay of S-nitrosating and denitrosating enzymatic reactions support the hypothesis that SNOs regulate the mitochondrion through redox mechanisms.[16]
In a study evaluating the effects on ursodeoxycholic acid (UDCA) on bile flow and cirrhosis, NO was found in bile as SNOs, primarily GSNO. UDCA-stimulated biliary NO secretion was abolished by the inhibition of iNOS with L-NAME in isolated perfused livers and also in rat livers depleted of GSH with buthionine sulfoximine. Moreover, the biliary secretion of NO species was significantly diminished in UDCA-infused transport mutant [ATP–binding cassette C2/multidrug resistance–associated protein 2–deficient] rats, and this finding was consistent with the involvement of the glutathione carrier ABCC2/Mrp2 in the canalicular transport of GSNO. It was particularly noteworthy that in cultured normal rat cholangiocytes, GSNO activated protein kinase B, protected against apoptosis, and enhanced UDCA-induced ATP release to the medium.[17] Finally, they demonstrated that retrograde GSNO infusion into the common bile duct increased bile flow and biliary bicarbonate secretion. The study concluded that UDCA-induced biliary secretion of GSNO contributed to stimulating ductal secretion of bile.
Neuromodulator
GSNO, along with glutathione and oxidized glutathione (GSSG), have been found to bind to the glutamate recognition site of the NMDA and AMPA receptors (via their γ-glutamyl moieties), and may be endogenous neuromodulators.[18][19] At millimolar concentrations, they may also modulate the redox state of the NMDA receptor complex.[19]
References
- ↑ "Prevention and reversion of nonalcoholic steatohepatitis in OB/OB mice by S-nitroso-N-acetylcysteine treatment". J Am Coll Nutr 27 (2): 299–305. April 2008. doi:10.1080/07315724.2008.10719703. PMID 18689562.
- ↑ "Detection of S-nitrosothiols in biological fluids: a comparison among the most widely applied methodologies". J. Chromatogr. B 851 (1–2): 124–39. May 2007. doi:10.1016/j.jchromb.2006.09.031. PMID 17035104.
- ↑ "Reduction of S-nitrosoglutathione by human alcohol dehydrogenase 3 is an irreversible reaction as analysed by electrospray mass spectrometry". Eur. J. Biochem. 270 (6): 1249–56. March 2003. doi:10.1046/j.1432-1033.2003.03486.x. PMID 12631283.
- ↑ "S-Nitrosoglutathione is a substrate for rat alcohol dehydrogenase class III isoenzyme". Biochem. J. 331 (2): 659–68. April 1998. doi:10.1042/bj3310659. PMID 9531510.
- ↑ "The Janus face of alcohol dehydrogenase 3". Chem. Biol. Interact. 178 (1–3): 29–35. March 2009. doi:10.1016/j.cbi.2008.10.050. PMID 19038239.
- ↑ "Dissection of a hypoxia-induced, nitric oxide-mediated signaling cascade". Mol. Biol. Cell 20 (18): 4083–90. September 2009. doi:10.1091/mbc.E09-05-0362. PMID 19625446.
- ↑ "S-nitrosylation in cardiovascular signaling". Circ. Res. 106 (4): 633–46. March 2010. doi:10.1161/CIRCRESAHA.109.207381. PMID 20203313.
- ↑ "Balancing reactivity against selectivity: the evolution of protein S-nitrosylation as an effector of cell signaling by nitric oxide". Cardiovasc. Res. 75 (2): 210–9. July 2007. doi:10.1016/j.cardiores.2007.04.023. PMID 17524376.
- ↑ "Protein S-nitrosylation: purview and parameters". Nat. Rev. Mol. Cell Biol. 6 (2): 150–66. February 2005. doi:10.1038/nrm1569. PMID 15688001.
- ↑ Kone BC (August 2006). "S-Nitrosylation: Targets, Controls and Outcomes". Current Genomics 7 (5): 301–10. doi:10.2174/138920206778604340. http://www.benthamscience.com/contents-JCode-CG-Vol-00000007-Iss-00000005.htm#3130838.
- ↑ "Systemic plasma levels of nitrite/nitrate (NOx) reflect brachial flow-mediated dilation responses in young men and women". Clin. Exp. Pharmacol. Physiol. 34 (12): 1291–3. December 2007. doi:10.1111/j.1440-1681.2007.04715.x. PMID 17973870.
- ↑ "Testing endothelial vasomotor function: nitric oxide, a multipotent molecule". Circulation 108 (17): 2049–53. October 2003. doi:10.1161/01.CIR.0000089507.19675.F9. PMID 14581383.
- ↑ "Protection from experimental asthma by an endogenous bronchodilator". Science 308 (5728): 1618–21. June 2005. doi:10.1126/science.1108228. PMID 15919956. Bibcode: 2005Sci...308.1618Q..
- ↑ 14.0 14.1 14.2 "Acute effects of aerosolized S-nitrosoglutathione in cystic fibrosis". Am. J. Respir. Crit. Care Med. 165 (7): 922–6. April 2002. doi:10.1164/ajrccm.165.7.2105032. PMID 11934715. https://kuscholarworks.ku.edu/bitstream/1808/16125/1/JohnsonMicheal_AJRCCM_165%287%291.pdf.
- ↑ "Endogenous nitrogen oxides and bronchodilator S-nitrosothiols in human airways". Proc. Natl. Acad. Sci. U.S.A. 90 (23): 10957–61. December 1993. doi:10.1073/pnas.90.23.10957. PMID 8248198. Bibcode: 1993PNAS...9010957G.
- ↑ Piantadosi CA (March 2011). "Regulation of mitochondrial processes by protein S-nitrosylation". Biochim Biophys Acta 1820 (6): 712–21. doi:10.1016/j.bbagen.2011.03.008. PMID 21397666.
- ↑ "Biliary secretion of S-nitrosoglutathione is involved in the hypercholeresis induced by ursodeoxycholic acid in the normal rat". Hepatology 52 (2): 667–77. August 2010. doi:10.1002/hep.23709. PMID 20683964.
- ↑ Steullet, P.; Neijt, H.C.; Cuénod, M.; Do, K.Q. (2006). "Synaptic plasticity impairment and hypofunction of NMDA receptors induced by glutathione deficit: Relevance to schizophrenia". Neuroscience 137 (3): 807–819. doi:10.1016/j.neuroscience.2005.10.014. ISSN 0306-4522. PMID 16330153.
- ↑ 19.0 19.1 Varga, V.; Jenei, Zs.; Janáky, R.; Saransaari, P.; Oja, S. S. (1997). "Glutathione is an endogenous ligand of rat brain N-methyl-D-aspartate (NMDA) and 2-amino-3-hydroxy-5-methyl-4-isoxazolepropionate (AMPA) receptors". Neurochemical Research 22 (9): 1165–1171. doi:10.1023/A:1027377605054. ISSN 0364-3190. PMID 9251108.
for the synthesis of S-Nitrosoglutathione see Hart, T.W., 1985. Some observations concerning the S-nitroso and S-phenylsulphonyl derivatives of L-cysteine and glutathione. Tetrahedron Letters, 26(16), pp.2013-2016.
External links
- S-Nitrosoglutathione at the US National Library of Medicine Medical Subject Headings (MeSH)
 |
