Chemistry:Sodium trimetaphosphate
| |
| Names | |
|---|---|
| Other names
Sodium trimetaphosphate
| |
| Identifiers | |
3D model (JSmol)
|
|
| EC Number |
|
PubChem CID
|
|
| UNII | |
| |
| |
| Properties | |
| Na3P3O9 | |
| Molar mass | 305.885 g/mol |
| Appearance | colorless or white crystals |
| Density | 2.49 g/cm3 (anhydrous) 1.786 g/cm3 (hexahydrate) |
| Melting point | 53 °C (127 °F; 326 K) (hexahydrate, decomposes to anyhdrous) |
| 22 g/100 mL | |
| Solubility | insoluble in alcohol |
Refractive index (nD)
|
1.433 (hexahydrate) |
| Structure | |
| triclinic (hexahydrate) | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
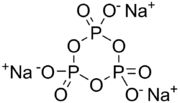
Sodium trimetaphosphate (also STMP), with formula Na3P3O9, is one of the metaphosphates of sodium. It has the formula Na
3P
3O
9 but the hexahydrate Na
3P
3O
9 · (H
2O)
6 is also well known. It is the sodium salt of trimetaphosphoric acid. It is a colourless solid that finds specialised applications in food and construction industries.[2]
Although drawn with a particular resonance structure, the trianion has high symmetry.[3]

3P
3O
9 · (H
2O)
6, as determined by X-ray crystallography. Highlighted is the P3O9 ring and some hydrogen bonding. Atoms are color coded as: purple = P, red = O, cyan = Na, white = H.[4]
Synthesis and reactions
Trisodium trimetaphosphate is produced industrially by heating sodium dihydrogen phosphate to 550 °C, a method first developed in 1955:[5]
- 3 NaH
2PO
4 → Na
3P
3O
9 + 3 H
2O
The trimetaphosphate dissolves in water and is precipitated by the addition of sodium chloride (common ion effect), affording the hexahydrate.[6] STMP can also prepared by heating samples of sodium polyphosphate,[2] or by a thermal reaction of orthophosphoric acid and sodium chloride at 600°C.[7][8]
- 3 NaH
3PO
4 + 3 NaCl → Na
3P
3O
9 + 3 H
2O + 3 HCl
Hydrolysis of the ring leads to the acyclic sodium triphosphate:
- Na3P3O9 + H2O → H2Na3P3O10
The analogous reaction of the metatriphosphate anion involves ring-opening by amine nucleophiles.[9]
References
- ↑ Lide, David R. (1998). Handbook of Chemistry and Physics (87 ed.). Boca Raton, Florida: CRC Press. pp. 4–86. ISBN 0-8493-0594-2.
- ↑ 2.0 2.1 Klaus Schrödter; Gerhard Bettermann; Thomas Staffel; Friedrich Wahl; Thomas Klein; Thomas Hofmann (2008). "Phosphoric Acid and Phosphates". Ullmann's Encyclopedia of Industrial Chemistry. Ullmann’s Encyclopedia of Industrial Chemistry. Weinheim: Wiley-VCH. doi:10.1002/14356007.a19_465.pub3. ISBN 978-3527306732.
- ↑ Greenwood, Norman N.; Earnshaw, Alan (1997). Chemistry of the Elements (2nd ed.). Butterworth-Heinemann. p. 530. ISBN 978-0-08-037941-8.
- ↑ Tordjman, I.; Durif, A.; Guitel, J. C. (1976). "Structure Cristalline du Trimétaphosphate de Sodium Hexahydraté: Na3P3O9*6H2O". Acta Crystallographica Section B Structural Crystallography and Crystal Chemistry 32 (6): 1871–1874. doi:10.1107/S0567740876006560. Bibcode: 1976AcCrB..32.1871T.
- ↑ Thilo, Erich; Grunze, Herbert (December 1955). "Zur Chemie der kondensierten Phosphate und Arsenate. XIII. Der Entwässerungsverlauf der Dihydrogenmonophosphate des Li, Na, K, und NH4.". Zeitschrift für anorganische und allgemeine Chemie 281 (5–6): 262–283. doi:10.1002/zaac.19552810504.
- ↑ Bell, R. N. (1950). "Sodium Metaphosphates". Inorganic Syntheses. 3. pp. 103–106. doi:10.1002/9780470132340.ch26. ISBN 9780470132340.
- ↑ Minh, Doan Pham; Ramaroson, Jocelyn; Nzihou, Ange; Sharrock, Patrick; Depelsenaire, Guy (1 January 2012). "A New Route for the Synthesis of Alkali Polyphosphate from Economical Starting Materials: Preparation and Characterization of Sodium Cyclotriphosphate". Phosphorus, Sulfur, and Silicon and the Related Elements 187 (1): 112–120. doi:10.1080/10426507.2011.590950. https://hal.archives-ouvertes.fr/hal-01634017/file/a-new-route-for-the-synthesis-of-alkali-polyphosphate.pdf.
- ↑ Pham Minh, Doan; Ramaroson, Jocelyn; Nzihou, Ange; Sharrock, Patrick (14 March 2012). "One-Step Synthesis of Sodium Trimetaphosphate (Na 3 P 3 O 9 ) from Sodium Chloride and Orthophosphoric Acid". Industrial & Engineering Chemistry Research 51 (10): 3851–3854. doi:10.1021/ie201085b. https://hal.archives-ouvertes.fr/hal-01632407/file/one-step-synthesis-of-sodium-trimetaphosphate.pdf.
- ↑ Bezold, Dominik; Dürr, Tobias; Singh, Jyoti; Jessen, Henning J. (2020). "Cyclotriphosphate: A Brief History, Recent Developments, and Perspectives in Synthesis". Chemistry – A European Journal 26 (11): 2298–2308. doi:10.1002/chem.201904433. PMID 31637774.
Template:Inorganic-compound-stub
 |

