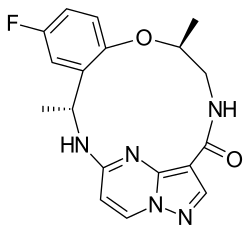
Chemistry:Repotrectinib
 | |
 | |
| Clinical data | |
|---|---|
| Trade names | Augtyro |
| Other names | TPX-0005 |
| AHFS/Drugs.com | Augtyro |
| License data | |
| Routes of administration | By mouth |
| Drug class | Tyrosine kinase inhibitor |
| ATC code |
|
| Legal status | |
| Legal status | |
| Identifiers | |
| CAS Number | |
| PubChem CID | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEBI | |
| ChEMBL | |
| PDB ligand | |
| Chemical and physical data | |
| Formula | C18H18FN5O2 |
| Molar mass | 355.373 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
Repotrectinib, sold under the brand name Augtyro, is an anti-cancer medication used for the treatment of non-small cell lung cancer.[1][2] It is taken by mouth.[1] Repotrectinib is an inhibitor of proto-oncogene tyrosine-protein kinase ROS1 (ROS1) and of the tropomyosin receptor tyrosine kinases (TRKs) TRKA, TRKB, and TRKC.[1]
The most common adverse reactions include dizziness, dysgeusia, peripheral neuropathy, constipation, dyspnea, ataxia, fatigue, cognitive disorders, and muscular weakness.[2]
Repotrectinib was approved for medical use in the United States in November 2023.[2][3]
Medical uses
Repotrectinib is indicated for the treatment of adults with locally advanced or metastatic ROS1-positive non-small cell lung cancer.[1][2]
History
Approval by the US Food and Drug Administration (FDA) was based on TRIDENT-1, a global, multicenter, single-arm, open-label, multi-cohort clinical trial (NCT03093116) which included participants with ROS1-positive locally advanced or metastatic non-small cell lung cancer.[2] Efficacy was evaluated in 71 ROS1 tyrosine kinase inhibitor-naïve participants who received up to one prior line of platinum-based chemotherapy and/or immunotherapy and 56 participants who received one prior ROS1 tyrosine kinase inhibitor with no prior platinum-based chemotherapy or immunotherapy.[2]
The FDA granted the application for repotrectinib priority review, breakthrough therapy, and fast track designations.[2]
References
- ↑ 1.0 1.1 1.2 1.3 1.4 "Augtyro- repotrectinib capsule". 15 November 2023. https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=fb526827-40ba-4462-94cf-179ae3b0cb8a.
- ↑ 2.0 2.1 2.2 2.3 2.4 2.5 2.6 "FDA approves repotrectinib for ROS1-positive non-small cell lung cancer". 15 November 2023. https://www.fda.gov/drugs/resources-information-approved-drugs/fda-approves-repotrectinib-ros1-positive-non-small-cell-lung-cancer.
 This article incorporates text from this source, which is in the public domain.
This article incorporates text from this source, which is in the public domain.
- ↑ "U.S. Food and Drug Administration Approves Augtyro (repotrectinib), a Next-Generation Tyrosine Kinase Inhibitor (TKI), for the Treatment of Locally Advanced or Metastatic ROS1-Positive Non-Small Cell Lung Cancer (NSCLC)" (Press release). Bristol Myers Squibb. 16 November 2023. Archived from the original on 16 November 2023. Retrieved 17 November 2023 – via Business Wire.
Further reading
- "Repotrectinib (TPX-0005) Is a Next-Generation ROS1/TRK/ALK Inhibitor That Potently Inhibits ROS1/TRK/ALK Solvent- Front Mutations". Cancer Discovery 8 (10): 1227–1236. October 2018. doi:10.1158/2159-8290.CD-18-0484. PMID 30093503.
External links
- "Repotrectinib (Code C133821)". 25 September 2023. https://ncit.nci.nih.gov/ncitbrowser/ConceptReport.jsp?dictionary=NCI%20Thesaurus&code=C133821.
- Clinical trial number NCT03093116 for "A Study of Repotrectinib (TPX-0005) in Patients With Advanced Solid Tumors Harboring ALK, ROS1, or NTRK1-3 Rearrangements (TRIDENT-1)" at ClinicalTrials.gov
 |

