Chemistry:Strukturbericht designation
In crystallography, a Strukturbericht designation or Strukturbericht type is a system of detailed crystal structure classification by analogy to another known structure. The designations were intended to be comprehensive but are mainly used as supplement to space group crystal structures designations, especially historically.[1][2] Each Strukturbericht designation is described by a single space group, but the designation includes additional information about the positions of the individual atoms, rather than just the symmetry of the crystal structure. While Strukturbericht symbols exist for many of the earliest observed and most common crystal structures, the system is not comprehensive, and is no longer being updated. Modern databases such as Inorganic Crystal Structure Database index thousands of structure types directly by the prototype compound (i.e. "the NaCl structure" instead of "the B1 structure").[3] These are essentially equivalent to the old Stukturbericht designations.
History
The designations were established by the journal Zeitschrift für Kristallographie – Crystalline Materials, which published its first round of supplemental reviews under the name Strukturbericht from 1913-1928.[4] These reports were collected into a book published in 1931 by Paul Peter Ewald and Carl Hermann which became Volume 1 of Strukturbericht.[5] While the series was continued after the war under the name Structure reports, which was published through 1990,[6] the series stopped generating new symbols. Instead, some new additional designations were given in books by Smithels,[7] and Pearson.[8]
For the first volume, the designation consisted of a capital letter (A,B,C,D,E,F,G,H,L,M,O) specifying a broad category of compounds, and then a number to specify a particular crystal structure. In the second volume, subscript numbers were added, some early symbols were modified (e.g. what was initially D1 became D01, noted in the tables below as "D1 → D01"), and the categories were modified (types I,K,S were added). In the third volume, the class I was renamed J. Later designations began to use a lower case letter in subscripts as well.[9]
A-compounds
The 'A' compounds are reserved for structures made up of atoms of all the same chemical element.
| Strukturbericht designation | Diagram | Prototype | Space group | Description |
|---|---|---|---|---|
| A1 | 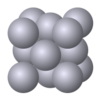
|
Cu | Fm3m | Cubic close-packed structure (also: Face-centered cubic structure) |
| A2 | File:BCC crystal structure.svg | W | Im3m | Body-centered cubic structure |
| A3 | File:HCP crystal structure.svg | Mg | P63/mmc | Hexagonal close-packed structure |
| A3' | 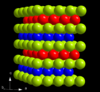
|
α-La | P63/mmc | α-La structure
(ABAC Barlow packing) |
| A4 | C (diamond) | Fd3m | Diamond cubic structure | |
| A5 | β-Sn | I41/amd | ||
| A6 | 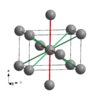
|
Indium | I4/mmm | Indium structure |
| A7 | 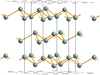
|
α-As | R3m | |
| A8 | 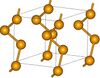
|
gray Se | P3121 | Also called γ-Se, but that term is also used for a monoclinic form. |
| A9 | 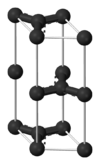
|
C (graphite) | P63/mmc | Hexagonal graphite structure |
| A10 | α-Hg | R3m | ||
| A11 | 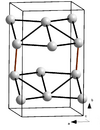
|
α-Ga | Cmca | α-Gallium structure |
| A12 | 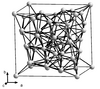
|
α-Mn | I43m | α-Manganese structure |
| A13 | 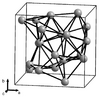
|
β-Mn | P4132 | β-Manganese structure |
| A14 | 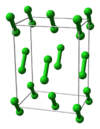
|
I2 | Cmca | Molecular iodine structure |
| A15 | 
|
β-W | Pm3n | Weaire–Phelan structure |
| A16 | α-S | Fddd | ||
| A17 | 
|
P | Cmca | Black phosphorus structure |
| A18 | Cl | P42/ncm | Incorrect structure[10] | |
| A19 → Ai, Ah | Po (incorrectly) | C2 | ||
| A20 | 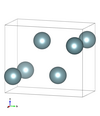
|
α-U | Cmcm | |
| Aa | α-Pa | I4/mmm | ||
| Ab | β-U | P42/mnm | σ phase | |
| Ac | α-Np | Pnma | ||
| Ad | β-Np | P4/nmm | ||
| Af | γ-HgSn6-10 | P6/mmm | ||
| Ag | B | P42/nnm | ||
| Ah | 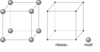
|
α-Po | Pm3m | Simple cubic structure |
| Ai | β-Po | R3m | ||
| Ak | γ-monoclinic Se | P21/c | ||
| Al | β-monoclinic Se | P21/c |
B-compounds
'B' designates compounds of two elements with equal numbers of atoms.
| Strukturbericht designation | Diagram | Prototype | Space group | Description |
|---|---|---|---|---|
| B1 | 
|
NaCl | Fm3m | Rock salt structure |
| B2 | 
|
CsCl | Pm3m | caesium chloride structure |
| B3 | 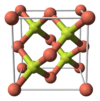
|
ZnS | F43m | Zincblende structure |
| B4 | 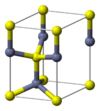
|
ZnS | P63mc | Wurtzite crystal structure |
| B5 | 4H SiC | P63mc | Moissanite (4H polytype) structure | |
| B6 | 6H SiC | P63mc | Moissanite(6H polytype) structure | |
| B7 | Si-C 15R | R3m | 15R-SiC structure | |
| B8 → B81 | ||||
| B81 | 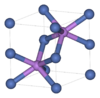
|
NiAs | P63/mmc | Nickeline structure |
| B82 | Ni2In | P63/mmc | ||
| B9 | 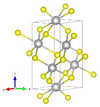
|
α-HgS | P3221 | Cinnabar structure |
| B10 | 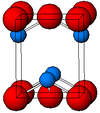
|
PbO | P4/nmm | Lead(II) oxide/Litharge structure |
| B11 | γ-CuTi | P4/nmm | ||
| B12 → Bk | BN (incorrectly)[11] | P63mc | Originally reported boron nitride structure | |
| B13 | 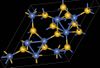
|
β-NiS | R3m | β-Nickel sulfide/Millerite structure |
| B14 | FeAs | Pnma | Westerveldite structure | |
| B15 → B27 | ||||
| B16 | 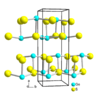
|
GeS | Pnma | Germanium(II) sulfide/Herzenbergite structure |
| B17 | 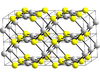
|
PtS | P42/mmc | Platinum(II) sulfide/Cooperite structure |
| B18 | CuS | P63/mmc | Copper monosulfide/Covellite structure | |
| B19 | β′-AuCd | Pmma | The martensitic β′-AuCd structure | |
| B20 | FeSi | P213 | FeSi/Fersilicite structure | |
| B21 | α-CO | P213 | α-Carbon monoxide structure | |
| B24 | TlF-II (incorrectly)[12] | Fmmm | Originally reported Thallium(I) fluoride structure | |
| B26 | 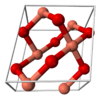
|
CuO | C2/c | Copper(II) oxide/Tenorite structure |
| B27 | 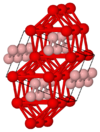
|
FeB | Pnma | Iron boride structure |
| B28 → B20 | ||||
| B29 → B16 | SnS (incorrectly) | Pnma | Tin(II) sulfide structure | |
| B30 | MgZn | Imm2 | ||
| B31 → B14 | MnP | Pnma | ||
| B32 | NaTl | Fd3m | ||
| B33 | 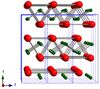
|
CrB | Cmcm | Chromium(III) boride structure |
| B34 | 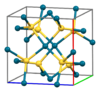
|
PdS | P42/m | Palladium(II) sulfide structure |
| B35 | CoSn | P6/mmm | ||
| B37 | SeTl | I4/mcm | ||
| Ba | CoU | I213 | ||
| Bb | ζ-AgZn | P3 | ||
| Bc → B33 | CaSi | Cmcm | ||
| Bd | η-NiSi | |||
| Be | CdSb | Pbca | ||
| Bf → B33 | CrB | (duplicate of B33) | ||
| Bg | MoB | I41/amd | Molybdenum boride structure | |
| Bh | 
|
WC | P6m2 | Tungsten carbide structure |
| Bi | AsTi | P63/mmc | ||
| Bk | 
|
BN | P63/mmc | Boron nitride structure |
| Bl | AsS | |||
| Bm | TiB |
C-compounds
'C' designates compounds of the stoichiometry AB2.
| Strukturbericht designation | Diagram | Prototype | Space group | Description |
|---|---|---|---|---|
| C1 | 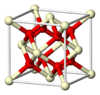
|
CaF2 | Fm3m | Fluorite structure |
| C1b | AgAsMg | F43m | Half Heusler structure | |
| C2 | 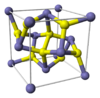
|
FeS2 | Pa3 | Pyrite structure |
| C3 | 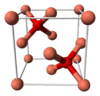
|
Cu2O | Pn3m | Cuprite structure |
| C4 | 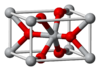
|
TiO2 | P42/mnm | Rutile structure |
| C5 | 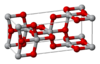
|
TiO2 | I41/amd | Anatase structure |
| C6 | 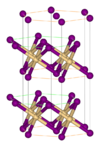
|
CdI2 | P3m1 | Cadmium iodide structure |
| C7 | 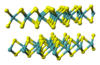
|
MoS2 | P63/mmc | Molybdenum disulfide structure |
| C8 | 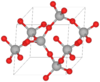
|
β-SiO2 | P6222 | β-Quartz structure |
| C9 | 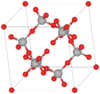
|
β-Cristobalite | Fd3m | Idealized β-Cristobalite structure |
| C10 | 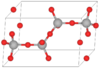
|
β-Tridymite | P63/mmc | β-Tridymite structure |
| C11 → C11a,C11b | ||||
| C11a | CaC2 | I4/mmm | ||
| C11b | 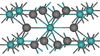
|
MoSi2 | I4/mmm | Molybdenum disilicide structure |
| C12 | CaSi2 | R3m | ||
| C13 | HgI2 | P42/nmc | Coccinite structure | |
| C14 | 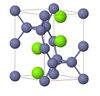
|
MgZn2 | P63/mmc | MgZn2 Laves phase |
| C15 | 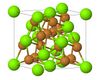
|
MgCu2 | Fd3m | MgCu2 Laves phase |
| C15b | AuBe5 | F43m | ||
| C16 | 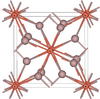
|
CuAl2 | I4/mcm | Khatyrkite structure |
| C18 | FeS2 | Pnnm | Marcasite structure | |
| C19 | 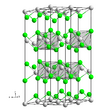
|
α-Sm | R3m | α-Samarium structure
(ABCBCACAB Barlow packing) |
| C21 | 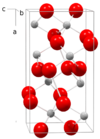
|
TiO2 | Pbca | Brookite structure |
| C22 | Fe2P | P321 (original, incorrect structure) P62m (revised) |
||
| C23 | 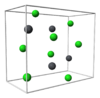
|
PbCl2 | Pnma | Cotunnite structure |
| C24 | 
|
HgBr2 | Cmc21 | Mercury(II) bromide structure |
| C25 → C28 | 
|
HgCl2 | Pnma | Mercury(II) chloride structure |
| C27 | CdI2 | P63mc | ||
| C28 | HgCl2 | |||
| C29 → C23 | SrH2 | Pnma | Strontium hydride structure | |
| C30 | 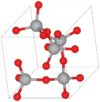
|
SiO2 | P41212 | α–Cristobalite structure |
| C32 | AlB2 | P6/mmm | Aluminium diboride structure | |
| C33 | 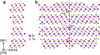
|
Bi2Te3 | R3m | Bismuth telluride structure |
| C34 | AuTe2 | C2/m | ||
| C35 | 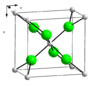
|
CaCl2 | Pnnm | Calcium chloride structure |
| C36 | 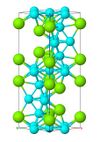
|
MgNi2 | P63/mmc | MgNi2 Laves phase |
| C37 | Co2Si | Pnma | ||
| C38 | Cu2Sb | P4/nmm | ||
| C39 → C15 | ||||
| C40 | CrSi2 | P6222 | ||
| C41 → C14 | ||||
| C42 | 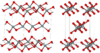
|
SiS2 | Ibam | Silicon disulfide structure |
| C43 | 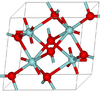
|
ZrO2 | P21/c | Baddeleyite structure |
| C44 | GeS2 | Fdd2 | Germanium disulfide structure | |
| C46 | 
|
AuTe2 | Pma2 | Krennerite structure |
| C47 | 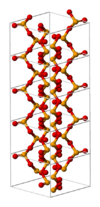
|
SeO2 | P42/mbc | Selenium dioxide structure |
| C48 → C11b | ||||
| C49 | ZrSi2 | Cmcm | ||
| C50 | 
|
PdCl2 | Pnnm | α-Palladium(II) chloride structure |
| C51 → C16 | ||||
| C53 | Sr Br2 | P4/n | ||
| C54 | 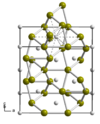
|
TiSi2 | Fddd | Titanium disilicide structure |
| Ca | Mg2Ni | P6222 | ||
| Cb | CuMg2 | Fddd | ||
| Cc | ThSi2 | I41/amd | ||
| Ce | PdSn2 | Aba2 | ||
| Cg | ThC2 | C2/c | ||
| Ch | Cu2Te | P6/mmm | ||
| Ck | LiZn2 |
D-compounds
'D' designates compounds of arbitrary stoichiometry. Originally, D1-D10 were set aside for stoichiometry AB3, D11-D20 for stoichiometry ABn for n > 3, D31-D50 for (ABn)2, and D51 up for the AmBn for arbitrary m and n.[9]
| Strukturbericht designation | Diagram | Prototype | Space group | Description |
|---|---|---|---|---|
| D02 | 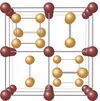
|
CoAs3 | Im3 | Skutterudite structure |
| D03 | BiF3 | Fm3m | Bismuth trifluoride structure | |
| D04 | CrCl3 | P3112 | Chromium(III) chloride structure | |
| D05 | 
|
BiI3 | R3 | Bismuth(III) iodide structure |
| D06 | 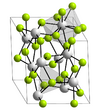
|
LaF3 | P3c1 | Lanthanum trifluoride/Fluocerite structure |
| D09 | 
|
α-ReO3 | Pm3m | α-Rhenium trioxide structure |
| D011 | 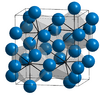
|
Fe3C | Pnma | Cementite structure |
| D012 | FeF3 | R3c | ||
| D014 → D012 | 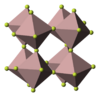
|
AlF3 | R32 | Aluminium fluoride structure |
| D015 | 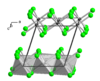
|
AlCl3 | C2/m | Aluminium chloride structure |
| D017 | BaS3 | P421m | ||
| D018 | File:Na3AsOffsetLayer.tif | Na3As | P63/mmc | Sodium arsenide structure |
| D019 | Ni3Sn | P63/mmc | ||
| D020 | Al3Ni | Pnma | ||
| D021 | 
|
Cu3P | P3c1 (original structure) P63cm (revised) |
Copper(I) phosphide structure |
| D022 | Al3Ti | I4/mmm | ||
| D023 | Al3Zr | I4/mmm | ||
| D024 | Ni3Ti | P63/mmc | ||
| D0a | β-TiCu3 | Pmmn | ||
| D0c → D0'c | SiU3 | I4/mcm | ||
| D0'c | Ir3Si | I4/mcm | ||
| D0d | AsMn3 | Cmcm | ||
| D0e | Ni3P | I4 | ||
| D1 | NH3 | P213 | ||
| D12 | SiF4 | I43m | Silicon tetrafluoride structure | |
| D13 | Al4Ba | I4/mmm | ||
| D1a | Ni4Mo | I4/m | ||
| D1b | Al4U | Imma | ||
| D1c | PtSn4 | Aba2 | ||
| D1d | Pb4Pt | P4/nbm | ||
| D1e | B4Th | P4/mbm | B4Th structure | |
| D1f → Cb | Mn4B | |||
| D1g | B4C | |||
| D2 → D02 | ||||
| D21 | 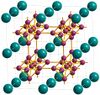
|
CaB6 | Pm3m | Calcium hexaboride structure |
| D23 | NaZn13 | Fm3c | ||
| D2b | Mn12Th | I4/mmm | ||
| D2c | MnU6 | I4/mcm | ||
| D2d | CaCu5 | P6/mmm | ||
| D2e | BaHg11 | Pm3 | ||
| D2f | UB12 | Fm3m | ||
| D2g | Fe8N | I4/mmmm | ||
| D2h | Al6Mn | Cmcm | ||
| D31 | Hg2Cl2 | I4/mmm | Calomel structure | |
| D51 | 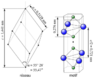
|
Al2O3 | R3c | Corrundum structure |
| D52 | La2O3 | P3m1 | ||
| D53 | 
|
Mn2O3 | Ia3 | Manganese(III) oxide/Bixbyite structure |
| D54 | Sb2O3 | Fd3m | ||
| D55 | Ag2O3 | Pn3m | ||
| D58 | 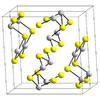
|
Sb2S3 | Pnma | Antimony trisulfide/Stibnite structure |
| D59 | Zn3Pt2 | P42/nmc | ||
| D510 | Cr3C2 | Pnma | ||
| D511 | Sb2O3 | Pccn | Antimony trioxide/Valentinite structure | |
| D512 | β–Bi2O3 | |||
| D513 | Al3Ni2 | P3m1 | ||
| D5a | Si2U3 | P4/mbm | ||
| D5b | Pt2Sn3 | P63/mmc | ||
| D5c | Pu2C3 | I43d | ||
| D5e | Ni3S2 | R32 | Nickel subsulfide/Heazlewoodite structure | |
| D5f | 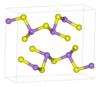
|
As2S3 | P21/n | Arsenic trisulfide structure |
| D71 | Al4C3 | R3m | Aluminium carbide structure | |
| D72 | Co3S4 | Fd3m | ||
| D73 | Th3P4 | I43d | Th3P4 structure | |
| D7a | 𝛿-Ni3Sn4 | |||
| D7b | Ta3B4 | Immm | ||
| D81 | Fe3Zn10 | I43m | ||
| D82 | Cu5Zn8 | I43m | Gamma brass structure | |
| D83 | Cu9Al4 | P43m | ||
| D84 | Cr23C6 | Fm3m | Cr23C6 crystal structure/Chromium(II) carbide structure | |
| D85 | Fe7W6 | R3m | ||
| D86 | Cu15Si4 | I43d | ||
| D88 | Mn5Si3 | P63/mcm | ||
| D89 | Co9S8 | Fm3m | ||
| D810 | Cr5Al8 | R3m | ||
| D811 | Co2Al5 | P63/mmc | ||
| D8a | Mn23Th6,Cu16Mg6Si7
(G-phase) |
Fm3m | ||
| D8b | σ-CrFe | P42/mnm | ||
| D8c | Mg2Zn11 | Pm3 | ||
| D8d | Co2Al9 | P21/c | ||
| D8e | Mg32(Al,Zn)49 | Im3 | ||
| D8f | Ge7Ir3 | Im3m | ||
| D8g | Ga2Mg5 | Ibam | ||
| D8h | W2B5 | P63/mmc | ||
| D8i | Mo2B5 | R3m | ||
| D8k | Th7S12 | P63/m | ||
| D8l | Cr5B4 | I4/mcm | ||
| D8m | W5Si3 | I4/mcm | ||
| D101 | C3Cr7 | Pnma | ||
| D102 | Fe3Th7 | P63mc | ||
| D31 → D31 | ||||
| D51 → D51 | ||||
| D52 → D52 | ||||
| D61 → D61 | ||||
| D81 → D81 | ||||
| D82 → D82 | ||||
| D83 → D83 |
E to K compounds
Letters between 'E' and 'K' designate more complex compounds.
| Strukturbericht designation | Diagram | Prototype | Space group | Description |
|---|---|---|---|---|
| E01 | PbFCl | P4/nmm | Matlockite structure | |
| E03 | CdOHCl | P63mc | ||
| E05 | FeOCl | Iron oxychloride structure | ||
| E07 | 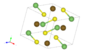
|
FeAsS | P21/c | Arsenopyrite structure |
| E11 | 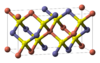
|
CuFeS2 | I42d | Chalcopyrite structure |
| E1a | MgCuAl2 | Cmcm | ||
| E1b | AgAuTe4 | P2/c | Sylvanite structure | |
| E21 | 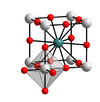
|
CaTiO3 | Pm3m | Perovskite structure |
| E22 | 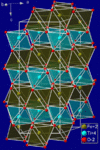
|
FeTiO3 | R3 | Ilmenite structure |
| E24 | NH4CdC13 | Pnma | ||
| E3 | CdAl2S4 | I4 | ||
| E33 | FeSb2S4 | Pnam | Berthierite structure | |
| E81 | Eu2Ir2O7 | Fd3m | Pyrochlore structure | |
| E91 | Ca3Al2O6 | Pm3m | ||
| E93 | Fe3W3C | Fd3m | ||
| E94 | Al5C3N | P63mc | ||
| E9a | Al7Cu2Fe | P4/mnc | ||
| E9b | Al8FeMg3Si6 | P62m | ||
| E9c | Mn3Al9Si | P63/mmc | ||
| E9d | AlLi3N2 | Ia3 | ||
| E9e | CuFe2S3 | Pnma | Cubanite structure | |
| F01 | NiSSb | P213 | Ullmannite structure | |
| F02 | COS | R3m | Carbonyl sulfide structure | |
| F51 | CrNaS2 | R3m | ||
| F52 | KN3 | I4/mcm | ||
| F54 | NH4O2Cl | P421m | ||
| F56 | CuSbS2 | Pnma | Chalcostibite structure | |
| F59 | KCNS | Pbcm | ||
| F510 | KAg(CN)2 | P31c | ||
| F513 | KBO2 | R3c | ||
| F5a | FeKS2 | C2/c | ||
| F51 → F51 | ||||
| F52 → F52 | ||||
| F61 → E11 | ||||
| G01 | 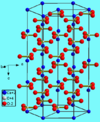
|
CaCO3 | R3c | Calcite structure |
| G02 | 
|
CaCO3 | Pmcn | Aragonite structure |
| G06 | KClO3 | P21/m | ||
| G3 | 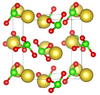
|
NaClO3 | P213 | Sodium chlorate structure |
| G32 | Na2SO3 | P3 | Sodium sulfite structure | |
| G4 → E22 | ||||
| G5 → E21 | ||||
| G71 | CeCO3F | P62c | Bastnäsite structure | |
| H01 | 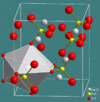
|
CaSO4 | Cmcm | Anhydrite structure |
| H02 | 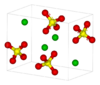
|
BaSO4 | Pnma | Baryte structure |
| H03 → S11 | ||||
| H05 | 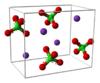
|
KClO4 | Pnma | Potassium perchlorate structure |
| H07 | BPO4 | I4 | ||
| H1 → H01 | ||||
| H11 | 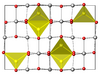
|
Al2MgO4 | Fd3m | Spinel structure |
| H12 → S12 | ||||
| H13 → S13 | ||||
| H15 | K2PtC14 | P4/mmmm | ||
| H16 | β-K2SO4 | Pnma | ||
| H2 → H02 | ||||
| H21 | 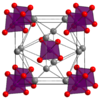
|
Ag3PO4 | P43n | Silver phosphate structure |
| H24 | Cu3S4V | P43m | ||
| H25 | AsCu3S4 | Pmn21 | Enargite structure | |
| H26 | Cu2FeS4Sn | I42m | Stannite structure | |
| H3 → S11 | ||||
| H31 → S14 | ||||
| H4 | File:CaWO4.tif | CaWO4 | I41/a | Scheelite structure |
| H57 | Ca5(PO4)3F,Cl,OH | P63/m | Apatite structure | |
| H11 → H11 | ||||
| H12 → S12 | ||||
| H13 → S13 | ||||
| H15 → H15 | ||||
| H31 → S14 | ||||
| H61 → J11 | ||||
| I11 → J11 | ||||
| I113 → J113 | ||||
| J11 | K2PtCl6 | Fm3m | ||
| J113 | K2GeF6 | P3m1 | ||
| K01 | K2S2O5 | P21/m | ||
| K11 | KSO3 | P321 | ||
| K12 | CsSO3 | P63mc | ||
| K31 | Cs3CoC15 | I4/mcm | ||
| K41 | NH4SO4 | P21/c |
L-compounds
'L' designates intermetallic compounds.
| Strukturbericht designation | Diagram | Prototype | Space group | Description |
|---|---|---|---|---|
| L10 | CuAu | P4/mmm | ||
| L11 | CuPt | R3m | ||
| L12 | 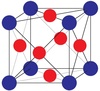
|
Cu3Au | Pm3m | Cu3Au structure |
| L13 | CdPt3 | Cmmm | ||
| L1a | CuPt3 | |||
| L21 | 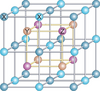
|
AlCu2Mn | Fm3m | Heusler compounds |
| L22 | Sb2Tl7 | Im3m | ||
| L2a | 𝛿-CuTi | P4/mmm | ||
| L60 | CuTi3 | P4/mmm | ||
| L'1 | Fe4N | Pm3m | ||
| L'12 | AlF3C | Perovskite structure | ||
| L'2 | ThH2 | I4/mmm | ||
| L'2b | ThH2 | I4/mmm | ||
| L'3 → B81 | Fe2N | |||
| L'32 | β-V2N | P31m | ||
| L60 | Ti3Cu | P4/mmm | ||
| L10 → L10 | ||||
| L'10 → L'1 | ||||
| L11 → L11 | ||||
| L12 → L12 | ||||
| L13 → L13 | ||||
| L20 → B2 | ||||
| L'20 → L'2 | ||||
| L21 → L21 | ||||
| L22 → L22 |
S-compounds
| Strukturbericht designation | Diagram | Prototype | Space group | Description |
|---|---|---|---|---|
| S11 | ZrSiO4 | I41/amd | Zircon structure | |
| S12 | 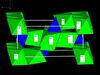
|
Mg2SiO4 | Pnma | Forsterite structure |
| S13 | 
|
Be2SiO4 | R3 | Phenakite structure |
| S14 | Al2Ca3Si3O12 | Ia3d | Garnet structure | |
| S21 | Sc2Si2O7 | C2/m | Thortveitite structure | |
| S31 | Be3Al2Si6O18 | P6/mcc | Beryl structure | |
| S32 | BaSi4O9 | P6c2 | ||
| S53 | Ca2MgSi2O7 | P421m | Åkermanite structure | |
| S6 | NaAlSi2O6H2O | I43d | ||
| S62 | Na8Al6Si6O24Cl2 | P43n |
See also
References
- ↑ "Index by Strukturbericht Designation". US Naval Laboratory Center for Materials Physics and Technology. 1 Sep 1995. http://aflowlib.duke.edu/users/egossett/lattice/struk/index.html.
- ↑ Mehl, Michael J.; Hicks, David; Toher, Cormac; Levy, Ohad; Hanson, Robert M.; Hart, Gus; Curtarolo, Stefano (2017). "The AFLOW Library of Crystallographic Prototypes: Part 1". Computational Materials Science 136: S1–S828. doi:10.1016/j.commatsci.2017.01.017. ISSN 0927-0256.
- ↑ Allmann, Rudolf; Hinek, Roland (2007-08-17). "The introduction of structure types into the Inorganic Crystal Structure Database ICSD". Acta Crystallographica Section A (International Union of Crystallography (IUCr)) 63 (5): 412–417. doi:10.1107/s0108767307038081. ISSN 0108-7673. PMID 17703075. Bibcode: 2007AcCrA..63..412A.
- ↑ Bragg, W. L. (1931). "Strukturbericht, 1913–1928". Nature 128 (3230): 511–512. doi:10.1038/128511a0. ISSN 0028-0836. Bibcode: 1931Natur.128..511B.
- ↑ Ewald, Paul Peter; Hermann, C. (1931). Zeitschrift für Kristallographie, Kristallgeometrie, Kristallphysik, Kristallchemie. Leipzig: Akademische Verlagsgesellschaft m.b.H..
- ↑ "International Union of Crystallography: Structure reports". https://www.iucr.org/publications/other/structure-reports.
- ↑ Smithells, Colin J (1949). Metals reference book (2nd ed.). New York: Interscience Publishers. OCLC 568858.
- ↑ Pearson, W. B. (1964). A handbook of lattice spacings and structures of metals and alloys. Oxford: Pergamon Press. doi:10.1016/C2013-0-08243-6. ISBN 978-1-4832-1318-7. OCLC 883896503.
- ↑ 9.0 9.1 Mehl, Michael J. (2019). "A Brief History of Strukturbericht Symbols and Other Crystallographic Classification Schemes". Journal of Physics: Conference Series (IOP Publishing) 1290 (1): 012016. doi:10.1088/1742-6596/1290/1/012016. ISSN 1742-6588. Bibcode: 2019JPhCS1290a2016M.
- ↑ Keesom, W.H.; Taconis, K.W. (1936). "On the crystal structure of chlorine". Physica (Elsevier BV) 3 (1–4): 237–242. doi:10.1016/s0031-8914(36)80226-2. ISSN 0031-8914. Bibcode: 1936Phy.....3..237K.
- ↑ PEASE, R. S. (1950). "Crystal Structure of Boron Nitride". Nature (Springer Science and Business Media LLC) 165 (4201): 722–723. doi:10.1038/165722b0. ISSN 0028-0836. PMID 15416803. Bibcode: 1950Natur.165..722P.
- ↑ Berastegui, P.; Hull, S. (2000). "The Crystal Structures of Thallium(I) Fluoride". Journal of Solid State Chemistry (Elsevier BV) 150 (2): 266–275. doi:10.1006/jssc.1999.8587. ISSN 0022-4596. Bibcode: 2000JSSCh.150..266B.
External links
- "Crystal Lattice Structures: Index by Strukturbericht Designation". https://www.atomic-scale-physics.de/lattice/struk/index.html.
- "Library of Crystallographic Prototypes - Strukturbericht Designations". http://aflowlib.org/CrystalDatabase/strukturberichts.html.
 |

