Chemistry:Infigratinib
 | |
| Clinical data | |
|---|---|
| Trade names | Truseltiq |
| Other names | BGJ-398 |
| AHFS/Drugs.com | Monograph |
| MedlinePlus | a621041 |
| License data |
|
| Pregnancy category | |
| Routes of administration | By mouth |
| Drug class | Tyrosine kinase inhibitor |
| ATC code | |
| Legal status | |
| Legal status | |
| Identifiers | |
| CAS Number | |
| PubChem CID | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| Chemical and physical data | |
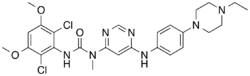
| Formula | C26H31Cl2N7O3 |
| Molar mass | 560.48 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
Infigratinib, sold under the brand name Truseltiq, is an anti-cancer medication used to treat cholangiocarcinoma (bile duct cancer).[1][4]
The most common side effects include increased phosphate level in the blood, increased creatinine levels in the blood, nail changes, mouth sores, dry eye, fatigue, alopecia, and palmar-plantar erythrodysesthesia (rash, redness, pain, swelling or blisters on the palms of the hands or soles of the feet).[5][6]
Infigratinib is a kinase inhibitor targeting the fibroblast growth factor receptors FGFR1, FGFR2, and FGFR3.[4][7]
Infigratinib was approved for medical use in the United States in May 2021.[4][5][6][8][9]
Medical uses
Infigratinib is indicated for the treatment of adults with previously treated, unresectable locally advanced or metastatic cholangiocarcinoma (bile duct cancer) with a fibroblast growth factor receptor 2 (FGFR2) fusion or other rearrangement as detected by an FDA-approved test.[4][5]
Adverse effects
The most common side effects include increased phosphate level in the blood, increased creatinine levels in the blood, nail changes, mouth sores, dry eye, fatigue, alopecia, and palmar-plantar erythrodysesthesia (rash, redness, pain, swelling or blisters on the palms of the hands or soles of the feet ).[5]
Infigratinib may cause serious side effects including detachment of retina (inner layer of the eye), increased phosphate level in the blood, and harm to an unborn baby.[5]
History
The US Food and Drug Administration (FDA) approved infigratinib based on evidence from one clinical trial (NCT02150967) of 108 participants with bile duct cancer (cholangiocarcinoma).[5] The CBGJ398X2204 trial was a multicenter open-label single-arm trial that enrolled 108 participants with previously treated, unresectable locally advanced or metastatic cholangiocarcinoma with an FGFR2 fusion or rearrangement as determined by local or central testing.[6] The trials were conducted at 18 sites in the United States, Europe, and Asia.[5] The trial enrolled adult participants with bile duct cancer who had been treated previously with chemotherapy for their advanced cancer and whose tumors had a certain type of abnormality in the FGFR2 gene.[5] Participants received infigratinib once daily by mouth for 21 consecutive days followed by 7 days off therapy.[5] This 28-day cycle was administered until disease progression or the side effects became too toxic.[5] The trial measured the percentage of participants who achieved partial or complete shrinkage of their cancer and how long that shrinkage lasted (duration of response or DoR).[5]
The FDA granted the application for infigratinib priority review, fast track, and orphan drug designations.[6]
Society and culture
Legal status
Infigratinib was designated an orphan drug by the FDA[10] and the European Medicines Agency in 2021.[11] It was approved for medical use under the FDA's accelerated approval program in May 2021.[5][6]
References
- ↑ 1.0 1.1 1.2 "Truseltiq". 22 November 2021. https://www.tga.gov.au/apm-summary/truseltiq.
- ↑ "Updates to the Prescribing Medicines in Pregnancy database". 12 May 2022. https://www.tga.gov.au/resources/resource/guidance/updates-prescribing-medicines-pregnancy-database.
- ↑ "Summary Basis of Decision (SBD) for Truseltiq". 23 October 2014. https://hpr-rps.hres.ca/reg-content/summary-basis-decision-detailTwo.php?linkID=SBD00561&lang=en.
- ↑ 4.0 4.1 4.2 4.3 4.4 "Truseltiq- infigratinib capsule". https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=af9a96cf-1cd7-8ace-e053-2a95a90a18cc.
- ↑ 5.00 5.01 5.02 5.03 5.04 5.05 5.06 5.07 5.08 5.09 5.10 5.11 "Drug Trials Snapshots: Truseltiq". 28 May 2021. https://www.fda.gov/drugs/drug-approvals-and-databases/drug-trials-snapshots-truseltiq.
 This article incorporates text from this source, which is in the public domain.
This article incorporates text from this source, which is in the public domain.
- ↑ 6.0 6.1 6.2 6.3 6.4 "FDA grants accelerated approval to infigratinib for metastatic cholang". 28 May 2021. https://www.fda.gov/drugs/resources-information-approved-drugs/fda-grants-accelerated-approval-infigratinib-metastatic-cholangiocarcinoma.
 This article incorporates text from this source, which is in the public domain.
This article incorporates text from this source, which is in the public domain.
- ↑ "Infigratinib (BGJ398): an investigational agent for the treatment of FGFR-altered intrahepatic cholangiocarcinoma". Expert Opinion on Investigational Drugs 30 (4): 309–316. April 2021. doi:10.1080/13543784.2021.1864320. PMID 33307867.
- ↑ "BridgeBio Pharma's Affiliate QED Therapeutics and Partner Helsinn Group Announce FDA Approval of Truseltiq (infigratinib) for Patients with Cholangiocarcinoma" (Press release). BridgeBio Pharma. 28 May 2021. Retrieved 28 May 2021 – via GlobeNewswire.
- ↑ (PDF) Advancing Health Through Innovation: New Drug Therapy Approvals 2021 (Report). 13 May 2022. https://www.fda.gov/media/155227/download. Retrieved 22 January 2023.
 This article incorporates text from this source, which is in the public domain.
This article incorporates text from this source, which is in the public domain.
- ↑ "Infigratinib Orphan Drug Designations and Approvals". 11 September 2019. https://www.accessdata.fda.gov/scripts/opdlisting/oopd/listResult.cfm.
- ↑ "EU/3/21/2475". 13 June 2022. https://www.ema.europa.eu/en/medicines/human/orphan-designations/eu-3-21-2475.
External links
- Clinical trial number NCT02150967 for "A Phase II, Single Arm Study of BGJ398 in Patients With Advanced Cholangiocarcinoma" at ClinicalTrials.gov
 |

