Chemistry:Sodium hyponitrite
| |
| Identifiers | |
|---|---|
3D model (JSmol)
|
|
| ChemSpider | |
PubChem CID
|
|
| |
| |
| Properties | |
| Na2N2O2 | |
| Molar mass | 105.99 g/mol |
| Appearance | colorless crystals |
| Density | 2.466 g/cm3 |
| Melting point | 100 °C (212 °F; 373 K) |
| Boiling point | 335 °C (635 °F; 608 K) decomposes |
| soluble | |
| Solubility | insoluble in ethanol |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
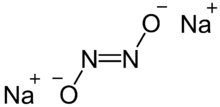
Sodium hyponitrite is a solid ionic compound with formula Na2N2O2 or (Na+)2[ON=NO]2−.[1]
There are cis and trans forms of the hyponitrite ion N2O2−2. The trans form is more common, but the cis form can be obtained too, and it is more reactive than the trans form.[1][2]
Trans isomer
The trans isomer is colorless and soluble in water and insoluble in ethanol and ether.[3][4]
Preparation
Sodium hyponitrite (trans) is conventionally prepared by reduction of sodium nitrite with sodium amalgam.[5][6][7]
- 2 NaNO2 + 4 Na(Hg) + 2 H2O → Na2N2O2 + 4 NaOH + 4 Hg
Sodium hyponitrite (trans) was prepared in 1927 by A. W. Scott by reacting alkyl nitrites, hydroxylammonium chloride, and sodium ethoxide[4][8]
- RONO + NH2OH + 2 EtONa → Na2N2O2 + ROH + 2 EtOH
An earlier method, published by D. Mendenhall in 1974, reacted gaseous nitric oxide (NO) with sodium metal in 1,2-dimethoxyethane, toluene, and benzophenone. The salt was then extracted with water.[9] The method was later modified to use pyridine[citation needed].
Other methods included oxidation of a concentrated solution of hydroxylamine with sodium nitrite in an alkaline medium[citation needed]; or electrolysis of sodium nitrite.[10]
Hydrates
A variety of hydrates Na2N2O2(H2O)x of the trans isomer have been reported, with x including 2, 3.5, 4, 5, 6, 7, 8, and 9;[11][3][12] but there is some dispute.[13]
The hydration water seems to be just trapped in the crystal lattice rather than coordinated to the ions.[13] The anhydrous substance can be obtained by drying the hydrates over phosphorus pentoxide and then heating them to 120 °C.[13]
Reactions
Sodium hyponitrite (trans) in solution is decomposed by carbon dioxide CO2 from air to form sodium carbonate.[14]
Liquid N2O4 oxidises sodium hyponitrite (trans) to give sodium peroxohyponitrite Na2+2[ON=NOO]2−).[15][1]
Cis isomer
The cis isomer of sodium hyponitrite is a white crystalline solid, insoluble in aprotic solvents, and (unlike the trans isomer) decomposed by water and other protic solvents.[2]
Preparation
The cis isomer of can be prepared by passing nitric oxide (NO) through a solution of sodium metal in liquid ammonia at −50 °C.[1]
The cis isomer was also obtained in 1996 by C. Feldmann and M. Jansen by heating sodium oxide Na2O with 77 kPa of nitrous oxide N2O (laughing gas) in a sealed tube at 360 °C for 2 hours. The two reagents combined to yield the cis hyponitrite quantitatively as white microcrystals.[8][2]
Properties and reactions
The anhydrous cis salt is stable up to 325 °C, when it disproportionates to nitrogen and sodium orthonitrite:[2]
- 3 Na2N2O2 → 2 Na3O(NO2) + 2 N2
It is generally more reactive than the trans isomer.[1]
See also
References
- ↑ 1.0 1.1 1.2 1.3 1.4 Egon Wiberg, Arnold Frederick Holleman (2001) Inorganic Chemistry, Elsevier ISBN:0-12-352651-5
- ↑ 2.0 2.1 2.2 2.3 Claus Feldmann, Martin Jansen (1996), "cis-Sodium Hyponitrite - A New Preparative Route and a Crystal Structure Analysis". Angewandte Chemie International Edition in English, volume 35, issue 15, pages 1728–1730. doi:10.1002/anie.199617281
- ↑ 3.0 3.1 Trambaklal Mohanlal Oza, Rajnikant Hariprasad Thaker (1955), "The Thermal Decomposition of Silver Hyponitrite". Journal of the American Chemical society, volume 77, issue 19, pages 4976–4980. doi:10.1021/ja01624a007
- ↑ 4.0 4.1 A. W. Scott (1927), "Sodium Hyponitrite". J. Am. Chem. Soc., volume = 49, issue 4, pages = 986–987. doi:10.1021/ja01403a502
- ↑ Addison, C. C.; Gamlen G. A.; Thompson, R. (1952). "70. The ultra-violet absorption spectra of sodium hyponitrite and sodium α-oxyhyponitrite : the analysis of mixtures with sodium nitrite and nitrate". J. Chem. Soc.: 338–345. doi:10.1039/jr9520000338.
- ↑ Neumann, R. C., Jr. Bussey, R. J. (1970). "High pressure studies. V. Activation volumes for combination and diffusion of geminate tert-butoxy radicals". J. Am. Chem. Soc. 92 (8): 2440–2445. doi:10.1021/ja00711a039.
- ↑ Greenwood, Norman N.; Earnshaw, Alan (1997). Chemistry of the Elements (2nd ed.). Butterworth-Heinemann. ISBN 978-0-08-037941-8.
- ↑ 8.0 8.1 Catherine E. Housecroft; Alan G. Sharpe (2008). "Chapter 15: The group 15 elements". Inorganic Chemistry (3rd ed.). Pearson. p. 468. ISBN 978-0-13-175553-6.
- ↑ G. David Mendenhall (1974), "Convenient synthesis of silver hyponitrite". Journal of the American Chemical society, volume 96, issue 15, page 5000. doi:10.1021/ja00822a054
- ↑ Polydoropoulos, C. N. Chem. Ind. (London) 1963, 1686 and references therein.
- ↑ James Riddick Partington and Chandulal Chhotalal Shah (1931), "Investigations on hyponitrites. Part I. Sodium hyponitrite: preparation and properties". Journal of the Chemical Society (Resumed), paper CCLXXXII, pages 2071-2080. doi:10.1039/JR9310002071
- ↑ C.N. Polydoropoulos, S.D. Voliotis (1967), "Sodium hyponitrite hexahydrate". Journal of Inorganic and Nuclear Chemistry, volume 29, issue 12, pages 2899–2901. doi:10.1016/0022-1902(67)80121-0
- ↑ 13.0 13.1 13.2 Gary L. Stucky, Jack L. Lambert, R. Dean Dragsdorf (1969), "The hydrates of sodium hyponitrite". Journal of Inorganic and Nuclear Chemistry, volume 31, issue 1, pages 29–32 doi:10.1016/0022-1902(69)80050-3
- ↑ Charlotte N. Conner, Caroline E. Donald, Martin N. Hughes, Christina Sami (1989), "The molar absorptivity of sodium hyponitrite". Polyhedron, volume 8, issue 21, pages 2621-2622. doi:10.1016/S0277-5387(00)81166-3
- ↑ M. N. Hughes and H. G. Nicklin (1969), "The action of dinitrogen tetroxide on sodium hyponitrite". Journal of the Chemical Society D: Chemical Communications, volume 1969, issue 2, page 80a. doi:10.1039/C2969000080A
 |

