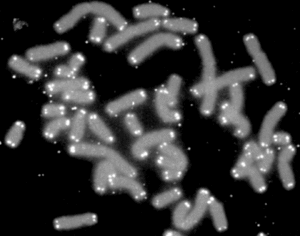
Biology:Telomere
A telomere (/ˈtɛləmɪər, ˈtiːlə-/; from grc τέλος (télos) 'end', and μέρος (méros) 'part') is a region of repetitive nucleotide sequences associated with specialized proteins at the ends of linear chromosomes (see Sequences). Telomeres are a widespread genetic feature most commonly found in eukaryotes. In most, if not all species possessing them, they protect the terminal regions of chromosomal DNA from progressive degradation and ensure the integrity of linear chromosomes by preventing DNA repair systems from mistaking the very ends of the DNA strand for a double-strand break.
Discovery
The existence of a special structure at the ends of chromosomes was independently proposed in 1938 by Hermann Joseph Muller, studying the fruit fly Drosophila melanogaster, and in 1939 by Barbara McClintock, working with maize.[1] Muller observed that the ends of irradiated fruit fly chromosomes did not present alterations such as deletions or inversions. He hypothesized the presence of a protective cap, which he coined "telomeres", from the Greek telos (end) and meros (part).[2]
In the early 1970s, Soviet theorist Alexei Olovnikov first recognized that chromosomes could not completely replicate their ends; this is known as the "end replication problem". Building on this, and accommodating Leonard Hayflick's idea of limited somatic cell division, Olovnikov suggested that DNA sequences are lost every time a cell replicates until the loss reaches a critical level, at which point cell division ends.[3][4][5] According to his theory of marginotomy DNA sequences at the ends of telomeres are represented by tandem repeats, which create a buffer that determines the number of divisions that a certain cell clone can undergo. Furthermore, it was predicted that a specialized DNA polymerase (originally called a tandem-DNA-polymerase) could extend telomeres in immortal tissues such as germ line, cancer cells and stem cells. It also followed from this hypothesis that organisms with circular genome, such as bacteria, do not have the end replication problem and therefore do not age.
In 1975–1977, Elizabeth Blackburn, working as a postdoctoral fellow at Yale University with Joseph G. Gall, discovered the unusual nature of telomeres, with their simple repeated DNA sequences composing chromosome ends.[6] Blackburn, Carol Greider, and Jack Szostak were awarded the 2009 Nobel Prize in Physiology or Medicine for the discovery of how chromosomes are protected by telomeres and the enzyme telomerase.[7]
Structure and function
End replication problem
During DNA replication, DNA polymerase cannot replicate the sequences present at the 3' ends of the parent strands. This is a consequence of its unidirectional mode of DNA synthesis: it can only attach new nucleotides to an existing 3'-end (that is, synthesis progresses 5'-3') and thus it requires a primer to initiate replication. On the leading strand (oriented 5'-3' within the replication fork), DNA-polymerase continuously replicates from the point of initiation all the way to the strand's end with the primer (made of RNA) then being excised and substituted by DNA. The lagging strand, however, is oriented 3'-5' with respect to the replication fork so continuous replication by DNA-polymerase is impossible, which necessitates discontinuous replication involving the repeated synthesis of primers further 5' of the site of initiation (see lagging strand replication). The last primer to be involved in lagging-strand replication sits near the 3'-end of the template (corresponding to the potential 5'-end of the lagging-strand). Originally it was believed that the last primer would sit at the very end of the template, thus, once removed, the DNA-polymerase that substitutes primers with DNA (DNA-Pol δ in eukaryotes)[note 1] would be unable to synthesize the "replacement DNA" from the 5'-end of the lagging strand so that the template nucleotides previously paired to the last primer would not be replicated.[8] It has since been questioned whether the last lagging strand primer is placed exactly at the 3'-end of the template and it was demonstrated that it is rather synthesized at a distance of about 70–100 nucleotides which is consistent with the finding that DNA in cultured human cell is shortened by 50–100 base pairs per cell division.[9]
If coding sequences are degraded in this process, potentially vital genetic code would be lost. Telomeres are non-coding, repetitive sequences located at the termini of linear chromosomes to act as buffers for those coding sequences further behind. They "cap" the end-sequences and are progressively degraded in the process of DNA replication.
The "end replication problem" is exclusive to linear chromosomes as circular chromosomes do not have ends lying without reach of DNA-polymerases. Most prokaryotes, relying on circular chromosomes, accordingly do not possess telomeres.[10] A small fraction of bacterial chromosomes (such as those in Streptomyces, Agrobacterium, and Borrelia), however, are linear and possess telomeres, which are very different from those of the eukaryotic chromosomes in structure and function. The known structures of bacterial telomeres take the form of proteins bound to the ends of linear chromosomes, or hairpin loops of single-stranded DNA at the ends of the linear chromosomes.[11]
Telomere ends and shelterin
At the very 3'-end of the telomere there is a 300 base pair overhang which can invade the double-stranded portion of the telomere forming a structure known as a T-loop. This loop is analogous to a knot, which stabilizes the telomere, and prevents the telomere ends from being recognized as breakpoints by the DNA repair machinery. Should non-homologous end joining occur at the telomeric ends, chromosomal fusion would result. The T-loop is maintained by several proteins, collectively referred to as the shelterin complex. In humans, the shelterin complex consists of six proteins identified as TRF1, TRF2, TIN2, POT1, TPP1, and RAP1.[12] In many species, the sequence repeats are enriched in guanine, e.g. TTAGGG in vertebrates,[13] which allows the formation of G-quadruplexes, a special conformation of DNA involving non-Watson-Crick base pairing. There are different subtypes depending on the involvement of single- or double-stranded DNA, among other things. There is evidence for the 3'-overhang in ciliates (that possess telomere repeats similar to those found in vertebrates) to form such G-quadruplexes that accommodate it, rather than a T-loop. G-quadruplexes present an obstacle for enzymes such as DNA-polymerases and are thus thought to be involved in the regulation of replication and transcription.[14]
Telomerase
Many organisms have an ribonucleoprotein enzyme called telomerase, which carries out the task of adding repetitive nucleotide sequences to the ends of the DNA. Telomerase "replenishes" the telomere "cap" and requires no ATP[15] In most multicellular eukaryotic organisms, telomerase is active only in germ cells, some types of stem cells such as embryonic stem cells, and certain white blood cells. Telomerase can be reactivated and telomeres reset back to an embryonic state by somatic cell nuclear transfer.[16] The steady shortening of telomeres with each replication in somatic (body) cells may have a role in senescence[17] and in the prevention of cancer.[18][19] This is because the telomeres act as a sort of time-delay "fuse", eventually running out after a certain number of cell divisions and resulting in the eventual loss of vital genetic information from the cell's chromosome with future divisions.[20][21]
Length
Telomere length varies greatly between species, from approximately 300 base pairs in yeast[22] to many kilobases in humans, and usually is composed of arrays of guanine-rich, six- to eight-base-pair-long repeats. Eukaryotic telomeres normally terminate with 3′ single-stranded-DNA overhang ranging from 75 to 300 bases, which is essential for telomere maintenance and capping. Multiple proteins binding single- and double-stranded telomere DNA have been identified.[23] These function in both telomere maintenance and capping. Telomeres form large loop structures called telomere loops, or T-loops. Here, the single-stranded DNA curls around in a long circle, stabilized by telomere-binding proteins.[24] At the very end of the T-loop, the single-stranded telomere DNA is held onto a region of double-stranded DNA by the telomere strand disrupting the double-helical DNA, and base pairing to one of the two strands. This triple-stranded structure is called a displacement loop or D-loop.[25]
Shortening
Oxidative damage
Apart from the end replication problem, in vitro studies have shown that telomeres accumulate damage due to oxidative stress and that oxidative stress-mediated DNA damage has a major influence on telomere shortening in vivo. There is a multitude of ways in which oxidative stress, mediated by reactive oxygen species (ROS), can lead to DNA damage; however, it is yet unclear whether the elevated rate in telomeres is brought about by their inherent susceptibility or a diminished activity of DNA repair systems in these regions.[26] Despite widespread agreement of the findings, widespread flaws regarding measurement and sampling have been pointed out; for example, a suspected species and tissue dependency of oxidative damage to telomeres is said to be insufficiently accounted for.[27] Population-based studies have indicated an interaction between anti-oxidant intake and telomere length. In the Long Island Breast Cancer Study Project (LIBCSP), authors found a moderate increase in breast cancer risk among women with the shortest telomeres and lower dietary intake of beta carotene, vitamin C or E.[28] These results [29] suggest that cancer risk due to telomere shortening may interact with other mechanisms of DNA damage, specifically oxidative stress.
Association with aging
Although telomeres shorten during the lifetime of an individual, it is telomere shortening-rate rather than telomere length that is associated with the lifespan of a species.[30] Critically short telomeres trigger a DNA damage response and cellular senescence.[30] Mice have much longer telomeres, but a greatly accelerated telomere shortening-rate and greatly reduced lifespan compared to humans and elephants.[31]
Telomere shortening is associated with aging, mortality, and aging-related diseases in experimental animals.[6][32] Although many factors can affect human lifespan, such as smoking, diet, and exercise, as persons approach the upper limit of human life expectancy, longer telomeres may be associated with lifespan.[33]
Potential effect of psychological stress
Meta-analyses found that increased perceived psychological stress was associated with a small decrease in telomere length—but that these associations attenuate to no significant association when accounting for publication bias. The literature concerning telomeres as integrative biomarkers of exposure to stress and adversity is dominated by cross-sectional and correlational studies, which makes causal interpretation problematic.[29][34] A 2020 review argued that the relationship between psychosocial stress and telomere length appears strongest for stress experienced in utero or early life.[35]
Lengthening
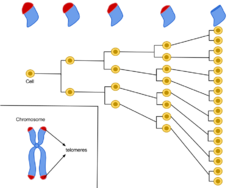
The phenomenon of limited cellular division was first observed by Leonard Hayflick, and is now referred to as the Hayflick limit.[36][37] Significant discoveries were subsequently made by a group of scientists organized at Geron Corporation by Geron's founder Michael D. West, that tied telomere shortening with the Hayflick limit.[38] The cloning of the catalytic component of telomerase enabled experiments to test whether the expression of telomerase at levels sufficient to prevent telomere shortening was capable of immortalizing human cells. Telomerase was demonstrated in a 1998 publication in Science to be capable of extending cell lifespan, and now is well-recognized as capable of immortalizing human somatic cells.[39]
Two studies on long-lived seabirds demonstrate that the role of telomeres is far from being understood. In 2003, scientists observed that the telomeres of Leach's storm-petrel (Oceanodroma leucorhoa) seem to lengthen with chronological age, the first observed instance of such behaviour of telomeres.[40]
A study reported that telomere length of different mammalian species correlates inversely rather than directly with lifespan, and concluded that the contribution of telomere length to lifespan remains controversial.[41] There is little evidence that, in humans, telomere length is a significant biomarker of normal aging with respect to important cognitive and physical abilities.[42]
Sequences
Experimentally verified and predicted telomere sequence motifs from more than 9000 species are collected in research community curated database TeloBase.[43] Some of the experimentally verified telomere nucleotide sequences are also listed in Telomerase Database website (see nucleic acid notation for letter representations).
| Group | Organism | Telomeric repeat (5' to 3' toward the end) |
|---|---|---|
| Vertebrates | Human, mouse, Xenopus | TTAGGG |
| Filamentous fungi | Neurospora crassa | TTAGGG |
| Slime moulds | Physarum, Didymium | TTAGGG |
| Dictyostelium | AG(1-8) | |
| Kinetoplastid protozoa | Trypanosoma, Crithidia | TTAGGG |
| Ciliate protozoa | Tetrahymena, Glaucoma | TTGGGG |
| Paramecium | TTGGG(T/G) | |
| Oxytricha, Stylonychia, Euplotes | TTTTGGGG | |
| Apicomplexan protozoa | Plasmodium | TTAGGG(T/C) |
| Higher plants | Arabidopsis thaliana | TTTAGGG |
| Cestrum elegans | TTTTTTAGGG[44] | |
| Allium | CTCGGTTATGGG[45] | |
| Green algae Chlamydomonas | TTTTAGGG | |
| Insects | Bombyx mori | TTAGG |
| Bombus terrestris | TTAGGTTGGGG[46] | |
| Vespula vulgaris | TTGCGTCTGGG[46] | |
| Roundworms | Ascaris lumbricoides | TTAGGC |
| Fission yeasts | Schizosaccharomyces pombe | TTAC(A)(C)G(1-8) |
| Budding yeasts | Saccharomyces cerevisiae | TGTGGGTGTGGTG (from RNA template) or G(2-3)(TG)(1-6)T (consensus) |
| Saccharomyces castellii | TCTGGGTG | |
| Candida glabrata | GGGGTCTGGGTGCTG | |
| Candida albicans | GGTGTACGGATGTCTAACTTCTT | |
| Candida tropicalis | GGTGTA[C/A]GGATGTCACGATCATT | |
| Candida maltosa | GGTGTACGGATGCAGACTCGCTT | |
| Candida guillermondii | GGTGTAC | |
| Candida pseudotropicalis | GGTGTACGGATTTGATTAGTTATGT | |
| Kluyveromyces lactis | GGTGTACGGATTTGATTAGGTATGT |
Research on disease risk
Preliminary research indicates that disease risk in aging may be associated with telomere shortening, senescent cells, or SASP (senescence-associated secretory phenotype).[30]
Measurement
Several techniques are currently employed to assess average telomere length in eukaryotic cells. One method is the Terminal Restriction Fragment (TRF) southern blot.[47][48] There is a Web-based Analyser of the Length of Telomeres (WALTER), software processing the TRF pictures.[49] A Real-Time PCR assay for telomere length involves determining the Telomere-to-Single Copy Gene (T/S) ratio, which is demonstrated to be proportional to the average telomere length in a cell.[50]
Tools have also been developed to estimate the length of telomere from whole genome sequencing (WGS) experiments. Amongst these are TelSeq,[51] Telomerecat[52] and telomereHunter.[53] Length estimation from WGS typically works by differentiating telomere sequencing reads and then inferring the length of telomere that produced that number of reads. These methods have been shown to correlate with preexisting methods of estimation such as PCR and TRF. Flow-FISH is used to quantify the length of telomeres in human white blood cells. A semi-automated method for measuring the average length of telomeres with Flow FISH was published in Nature Protocols in 2006.[54]
While multiple companies offer telomere length measurement services, the utility of these measurements for widespread clinical or personal use has been questioned.[55][56] Nobel Prize winner Elizabeth Blackburn, who was co-founder of one company, promoted the clinical utility of telomere length measures.[57]
In wildlife
During the last two decades, eco-evolutionary studies have investigated the relevance of life-history traits and environmental conditions on telomeres of wildlife. Most of these studies have been conducted in endotherms, i.e. birds and mammals. They have provided evidence for the inheritance of telomere length; however, heritability estimates vary greatly within and among species.[58] Age and telomere length often negatively correlate in vertebrates, but this decline is variable among taxa and linked to the method used for estimating telomere length.[59] In contrast, the available information shows no sex differences in telomere length across vertebrates.[60] Phylogeny and life history traits such as body size or the pace of life can also affect telomere dynamics. For example, it has been described across species of birds and mammals.[61] In 2019, a meta-analysis confirmed that the exposure to stressors (e.g. pathogen infection, competition, reproductive effort and high activity level) was associated with shorter telomeres across different animal taxa.[62]
Studies on ectotherms, and other non-mammalian organisms, show that there is no single universal model of telomere erosion; rather, there is wide variation in relevant dynamics across Metazoa, and even within smaller taxonomic groups these patterns appear diverse.[63]
See also
- Epigenetic clock
- Centromere
- DNA damage theory of aging
- Immortality
- Maximum life span
- Rejuvenation (aging)
- Senescence, biological aging
- Tankyrase
- Telomere-binding protein
- G-quartet
- Immortal DNA strand hypothesis
Notes
- ↑ During replication, multiple DNA-polymerases are involved.
References
- ↑ Varela, E.; Blasco, M. A. (March 2010). "2009 Nobel Prize in Physiology or Medicine: telomeres and telomerase" (in en). Oncogene 29 (11): 1561–1565. doi:10.1038/onc.2010.15. ISSN 1476-5594. PMID 20237481. https://www.nature.com/articles/onc201015.
- ↑ Muller, H.J. (1938). The Remaking of Chromosomes. Woods Hole. pp. 181–198.
- ↑ Olovnikov, A. M. (1971). "[Principle of marginotomy in template synthesis of polynucleotides"]. Doklady Akademii Nauk SSSR 201 (6): 1496–1499. ISSN 0002-3264. PMID 5158754. https://pubmed.ncbi.nlm.nih.gov/5158754/.
- ↑ Olovnikov, A. M. (1973-09-14). "A theory of marginotomy: The incomplete copying of template margin in enzymic synthesis of polynucleotides and biological significance of the phenomenon" (in en). Journal of Theoretical Biology 41 (1): 181–190. doi:10.1016/0022-5193(73)90198-7. ISSN 0022-5193. PMID 4754905. Bibcode: 1973JThBi..41..181O. https://dx.doi.org/10.1016/0022-5193%2873%2990198-7.
- ↑ Olovnikov, A. M. (1996). "Telomeres, telomerase, and aging: origin of the theory". Experimental Gerontology 31 (4): 443–448. doi:10.1016/0531-5565(96)00005-8. ISSN 0531-5565. PMID 9415101. https://pubmed.ncbi.nlm.nih.gov/9415101/.
- ↑ 6.0 6.1 "A tandemly repeated sequence at the termini of the extrachromosomal ribosomal RNA genes in Tetrahymena". Journal of Molecular Biology 120 (1): 33–53. March 1978. doi:10.1016/0022-2836(78)90294-2. PMID 642006.
- ↑ "Elizabeth H. Blackburn, Carol W. Greider, Jack W. Szostak: The Nobel Prize in Physiology or Medicine 2009". Nobel Foundation. 2009-10-05. http://nobelprize.org/nobel_prizes/medicine/laureates/2009/press.html.
- ↑ "A theory of marginotomy. The incomplete copying of template margin in enzymic synthesis of polynucleotides and biological significance of the phenomenon". Journal of Theoretical Biology 41 (1): 181–90. September 1973. doi:10.1016/0022-5193(73)90198-7. PMID 4754905. Bibcode: 1973JThBi..41..181O.
- ↑ "Early and late steps in telomere overhang processing in normal human cells: the position of the final RNA primer drives telomere shortening". Genes & Development 26 (11): 1167–1178. June 2012. doi:10.1101/gad.187211.112. PMID 22661228.
- ↑ Nelson, David L.; Lehninger, Albert L.; Cox, Michael M. (2008). Lehninger Principles of Biochemistry (5th ed.). New York: W.H. Freeman. ISBN 9780716771081. OCLC 191854286. https://archive.org/details/lehningerprincip00lehn_1.
- ↑ Maloy, Stanley (July 12, 2002). "Bacterial Chromosome Structure". http://www.sci.sdsu.edu/~smaloy/MicrobialGenetics/topics/chroms-genes-prots/chromosomes.html.
- ↑ "Role of shelterin in cancer and aging". Aging Cell 9 (5): 653–66. October 2010. doi:10.1111/j.1474-9726.2010.00596.x. PMID 20569239.
- ↑ "Conservation of the human telomere sequence (TTAGGG)n among vertebrates". Proceedings of the National Academy of Sciences of the United States of America 86 (18): 7049–53. September 1989. doi:10.1073/pnas.86.18.7049. PMID 2780561. Bibcode: 1989PNAS...86.7049M.
- ↑ "G-quadruplex structures: in vivo evidence and function". Trends in Cell Biology 19 (8): 414–22. August 2009. doi:10.1016/j.tcb.2009.05.002. PMID 19589679.
- ↑ "Telomerase Repeated Amplification Protocol (TRAP)". Bio-Protocol 5 (22): e1657. November 2015. doi:10.21769/bioprotoc.1657. PMID 27182535.
- ↑ "Extension of cell life-span and telomere length in animals cloned from senescent somatic cells". Science 288 (5466): 665–9. April 2000. doi:10.1126/science.288.5466.665. PMID 10784448. Bibcode: 2000Sci...288..665L.
- ↑ Whittemore, Kurt; Vera, Elsa; Martínez-Nevado, Eva; Sanpera, Carola; Blasco, Maria A. (2019). "Telomere shortening rate predicts species life span". Proceedings of the National Academy of Sciences 116 (30): 15122–15127. doi:10.1073/pnas.1902452116. ISSN 0027-8424. PMID 31285335. Bibcode: 2019PNAS..11615122W.
- ↑ "Senescence and immortalization: role of telomeres and telomerase". Carcinogenesis 26 (5): 867–74. May 2005. doi:10.1093/carcin/bgh296. PMID 15471900.
- ↑ "Telomeres, telomerase, and tumorigenesis--a review". MedGenMed 6 (3): 19. July 2004. PMID 15520642.
- ↑ "Telomeres, telomerase and senescence". BioEssays 12 (8): 363–9. August 1990. doi:10.1002/bies.950120803. PMID 2241933.
- ↑ Barnes, R.P., de Rosa, M., Thosar, S.A., et al., Telomeric 8-oxo-guanine drives rapid premature senescence in the absence of telomere shortening, Nature, June 30, 2022; Nat Struct Mol Biol 29, 639–652 (2022). https://doi.org/10.1038/s41594-022-00790-y
- ↑ "DNA sequences of telomeres maintained in yeast". Nature 310 (5973): 154–7. 1984. doi:10.1038/310154a0. PMID 6330571. Bibcode: 1984Natur.310..154S.
- ↑ "Characterization of the yeast telomere nucleoprotein core: Rap1 binds independently to each recognition site". The Journal of Biological Chemistry 285 (46): 35814–24. November 2010. doi:10.1074/jbc.M110.170167. PMID 20826803.
- ↑ "Mammalian telomeres end in a large duplex loop". Cell 97 (4): 503–14. May 1999. doi:10.1016/S0092-8674(00)80760-6. PMID 10338214.
- ↑ "Quadruplex DNA: sequence, topology and structure". Nucleic Acids Research 34 (19): 5402–15. 2006. doi:10.1093/nar/gkl655. PMID 17012276.
- ↑ "The impact of oxidative DNA damage and stress on telomere homeostasis". Mechanisms of Ageing and Development 177: 37–45. 2019. doi:10.1016/j.mad.2018.03.013. PMID 29604323.
- ↑ "Does oxidative stress shorten telomeres in vivo? A review". Biology Letters 13 (12): 20170463. December 2017. doi:10.1098/rsbl.2017.0463. PMID 29212750.
- ↑ "Telomere length, oxidative damage, antioxidants and breast cancer risk". International Journal of Cancer 124 (7): 1637–43. April 2009. doi:10.1002/ijc.24105. PMID 19089916.
- ↑ 29.0 29.1 "Perceived stress and telomere length: A systematic review, meta-analysis, and methodologic considerations for advancing the field". Brain, Behavior, and Immunity 54: 158–169. May 2016. doi:10.1016/j.bbi.2016.02.002. PMID 26853993.
- ↑ 30.0 30.1 30.2 "Telomere dysfunction in ageing and age-related diseases". Nature Cell Biology 24 (2): 135–147. 2022. doi:10.1038/s41556-022-00842-x. PMID 35165420.
- ↑ "Telomerase as a Therapeutic Target in Cardiovascular Disease". Arteriosclerosis, Thrombosis, and Vascular Biology 41 (3): 1047–1061. 2021. doi:10.1161/ATVBAHA.120.315695. PMID 33504179.
- ↑ "Divergence of sperm and leukocyte age-dependent telomere dynamics: implications for male-driven evolution of telomere length in humans". Molecular Human Reproduction 18 (11): 517–22. November 2012. doi:10.1093/molehr/gas028. PMID 22782639.
- ↑ "Telomeres and the natural lifespan limit in humans". Aging 9 (4): 130–1142. 2017. doi:10.18632/aging.101216. PMID 28394764.
- ↑ "Telomeres as integrative markers of exposure to stress and adversity: a systematic review and meta-analysis". Royal Society Open Science 5 (8): 180744. August 2018. doi:10.1098/rsos.180744. PMID 30225068. Bibcode: 2018RSOS....580744P.
- ↑ "Psychosocial Stressors and Telomere Length: A Current Review of the Science". Annual Review of Public Health 41: 223–245. April 2020. doi:10.1146/annurev-publhealth-040119-094239. PMID 31900099.
- ↑ "The serial cultivation of human diploid cell strains". Experimental Cell Research 25 (3): 585–621. December 1961. doi:10.1016/0014-4827(61)90192-6. PMID 13905658.
- ↑ "The limited in vitro lifetime of human diploid cell strains". Experimental Cell Research 37 (3): 614–36. March 1965. doi:10.1016/0014-4827(65)90211-9. PMID 14315085.
- ↑ "The RNA component of human telomerase". Science 269 (5228): 1236–41. September 1995. doi:10.1126/science.7544491. PMID 7544491. Bibcode: 1995Sci...269.1236F.
- ↑ "Extension of life-span by introduction of telomerase into normal human cells". Science 279 (5349): 349–52. January 1998. doi:10.1126/science.279.5349.349. PMID 9454332. Bibcode: 1998Sci...279..349B.
- ↑ "Measuring vertebrate telomeres: applications and limitations". Molecular Ecology 13 (9): 2523–33. September 2004. doi:10.1111/j.1365-294X.2004.02291.x. PMID 15315667. Bibcode: 2004MolEc..13.2523N. http://eprints.whiterose.ac.uk/353/1/burket16.pdf.
- ↑ "Comparative biology of mammalian telomeres: hypotheses on ancestral states and the roles of telomeres in longevity determination". Aging Cell 10 (5): 761–8. October 2011. doi:10.1111/j.1474-9726.2011.00718.x. PMID 21518243.
- ↑ "Telomere length and aging biomarkers in 70-year-olds: the Lothian Birth Cohort 1936". Neurobiology of Aging 33 (7): 1486.e3–8. July 2012. doi:10.1016/j.neurobiolaging.2010.11.013. PMID 21194798.
- ↑ Lyčka, Martin; Bubeník, Michal; Závodník, Michal; Peska, Vratislav; Fajkus, Petr; Demko, Martin; Fajkus, Jiří; Fojtová, Miloslava (2023-08-21). "TeloBase: a community-curated database of telomere sequences across the tree of life". Nucleic Acids Research 52 (D1): D311–D321. doi:10.1093/nar/gkad672. ISSN 0305-1048. PMID 37602392. PMC 10767889. https://doi.org/10.1093/nar/gkad672.
- ↑ "Characterisation of an unusual telomere motif (TTTTTTAGGG)n in the plant Cestrum elegans (Solanaceae), a species with a large genome". The Plant Journal 82 (4): 644–54. May 2015. doi:10.1111/tpj.12839. PMID 25828846.
- ↑ "Allium telomeres unmasked: the unusual telomeric sequence (CTCGGTTATGGG)n is synthesized by telomerase". The Plant Journal 85 (3): 337–47. February 2016. doi:10.1111/tpj.13115. PMID 26716914.
- ↑ 46.0 46.1 Fajkus, Petr; Adámik, Matej; Nelson, Andrew D L; Kilar, Agata M; Franek, Michal; Bubeník, Michal; Frydrychová, Radmila Čapková; Votavová, Alena et al. (2023-01-11). "Telomerase RNA in Hymenoptera (Insecta) switched to plant/ciliate-like biogenesis" (in en). Nucleic Acids Research 51 (1): 420–433. doi:10.1093/nar/gkac1202. ISSN 0305-1048. PMID 36546771. PMC 9841428. https://academic.oup.com/nar/article/51/1/420/6956357.
- ↑ "Human telomeres contain at least three types of G-rich repeat distributed non-randomly". Nucleic Acids Research 17 (12): 4611–27. June 1989. doi:10.1093/nar/17.12.4611. PMID 2664709.
- ↑ "Telomere length dynamics in human lymphocyte subpopulations measured by flow cytometry". Nature Biotechnology 16 (8): 743–7. August 1998. doi:10.1038/nbt0898-743. PMID 9702772.
- ↑ Lyčka, Martin; Peska, Vratislav; Demko, Martin; Spyroglou, Ioannis; Kilar, Agata; Fajkus, Jiří; Fojtová, Miloslava (December 2021). "WALTER: an easy way to online evaluate telomere lengths from terminal restriction fragment analysis" (in en). BMC Bioinformatics 22 (1): 145. doi:10.1186/s12859-021-04064-0. ISSN 1471-2105. PMID 33752601.
- ↑ "Telomere measurement by quantitative PCR". Nucleic Acids Research 30 (10): 47e–47. May 2002. doi:10.1093/nar/30.10.e47. PMID 12000852.
- ↑ "Estimating telomere length from whole genome sequence data". Nucleic Acids Research 42 (9): e75. 2014. doi:10.1093/nar/gku181. PMID 24609383.
- ↑ "Telomerecat: A ploidy-agnostic method for estimating telomere length from whole genome sequencing data.". Scientific Reports 8 (1): 1300. 2018. doi:10.1038/s41598-017-14403-y. PMID 29358629. Bibcode: 2018NatSR...8.1300F.
- ↑ "TelomereHunter–in silico estimation of telomere content and composition from cancer genomes.". BMC Bioinformatics 20 (1): 272. 2019. doi:10.1186/s12859-019-2851-0. PMID 31138115.
- ↑ "Flow cytometry and FISH to measure the average length of telomeres (flow FISH)". Nature Protocols 1 (5): 2365–76. December 2006. doi:10.1038/nprot.2006.263. PMID 17406480.
- ↑ Pollack, Andrew (May 18, 2011). "A Blood Test Offers Clues to Longevity". The New York Times. https://www.nytimes.com/2011/05/19/business/19life.html.
- ↑ "Will your telomeres tell your future?". BMJ 344: e1727. March 2012. doi:10.1136/bmj.e1727. PMID 22415954.
- ↑ "Spit test offers guide to health". Nature. 2011. doi:10.1038/news.2011.330.
- ↑ Dugdale, Hannah L.; Richardson, David S. (2018-01-15). "Heritability of telomere variation: it is all about the environment!". Philosophical Transactions of the Royal Society B: Biological Sciences 373 (1741): 20160450. doi:10.1098/rstb.2016.0450. ISSN 0962-8436. PMID 29335377. PMC 5784070. http://dx.doi.org/10.1098/rstb.2016.0450.
- ↑ Remot, Florentin; Ronget, Victor; Froy, Hannah; Rey, Benjamin; Gaillard, Jean-Michel; Nussey, Daniel H.; Lemaitre, Jean-François (2021-09-07). "Decline in telomere length with increasing age across nonhuman vertebrates: A meta-analysis". Molecular Ecology 31 (23): 5917–5932. doi:10.1111/mec.16145. ISSN 0962-1083. PMID 34437736. http://dx.doi.org/10.1111/mec.16145.
- ↑ Remot, Florentin; Ronget, Victor; Froy, Hannah; Rey, Benjamin; Gaillard, Jean-Michel; Nussey, Daniel H.; Lemaître, Jean-François (November 2020). "No sex differences in adult telomere length across vertebrates: a meta-analysis". Royal Society Open Science 7 (11): 200548. doi:10.1098/rsos.200548. ISSN 2054-5703. PMID 33391781. PMC 7735339. Bibcode: 2020RSOS....700548R. http://dx.doi.org/10.1098/rsos.200548.
- ↑ Pepke, Michael Le; Eisenberg, Dan T. A. (2021-03-16). "On the comparative biology of mammalian telomeres: Telomere length co-evolves with body mass, lifespan and cancer risk" (in en). Molecular Ecology 31 (23): 6286–6296. doi:10.1111/mec.15870. ISSN 0962-1083. PMID 33662151.
- ↑ Chatelain, Marion; Drobniak, Szymon M.; Szulkin, Marta (2019-11-27). "The association between stressors and telomeres in non-human vertebrates: a meta-analysis". Ecology Letters 23 (2): 381–398. doi:10.1111/ele.13426. ISSN 1461-023X. PMID 31773847.
- ↑ "Ectothermic telomeres: it's time they came in from the cold". Philosophical Transactions of the Royal Society of London. Series B, Biological Sciences 373 (1741): 20160449. March 2018. doi:10.1098/rstb.2016.0449. PMID 29335373.
External links
- Telomeres and Telomerase: The Means to the End Nobel Lecture by Elizabeth Blackburn, which includes a reference to the impact of stress, and pessimism on telomere length
- Telomerase and the Consequences of Telomere Dysfunction Nobel Lecture by Carol Greider
- DNA Ends: Just the Beginning Nobel Lecture by Jack Szostak
 |