Chemistry:Potassium tetraiodomercurate(II)
| |
| |
| Names | |
|---|---|
| IUPAC name
potassium tetraiodidomercurate(II)
| |
| Other names
potassium mercuric iodide,
Nessler's reagent (principal component) | |
| Identifiers | |
3D model (JSmol)
|
|
| ChEBI | |
| ChemSpider | |
| EC Number |
|
PubChem CID
|
|
| UNII | |
| UN number | 3287 |
| |
| |
| Properties | |
| K 2[HgI 4][1] | |
| Molar mass | 786.406 g·mol−1 |
| Appearance | yellow crystals |
| Odor | odorless |
| Density | 4.29 g/cm3 |
| very soluble | |
| Solubility | soluble in alcohol, ether, acetone |
| Hazards | |
| Safety data sheet | External MSDS for Nessler's reagent |
| Related compounds | |
Other anions
|
Mercury(II) iodide |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
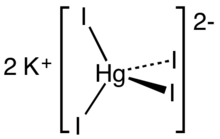
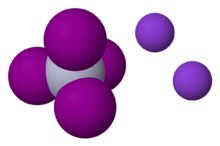
Potassium tetraiodomercurate(II) is an inorganic compound with the chemical formula K
2[[[Mercury (element)|Hg]]I
4]. It consists of potassium cations and tetraiodomercurate(II) anions. It is the active agent in Nessler's reagent, used for detection of ammonia.[2]
Preparation
The compound crystallizes from a heated solution of mercuric iodide, potassium iodide, and precisely 2% water in acetone. Attempted synthesis in concentrated aqueous solution will give the pale orange monohydrate K[Hg(H
2O)I
3] instead.[3]
Applications
K
2[HgI
4] is a precursor to analogous copper and silver salts M
2[HgI
4] (M=Cu, Ag).[4]
Nessler's reagent
Nessler's reagent, named after Julius Neßler (Nessler), is a 0.09 mol/L solution of potassium tetraiodomercurate(II) in 2.5 mol/L potassium hydroxide. This pale solution becomes deeper yellow in the presence of ammonia (NH
3). At higher concentrations, a brown precipitate derivative of Millon's base (HgO · Hg(NH
2)Cl) may form. The sensitivity as a spot test is about 0.3 μg NH
3 in 2 μL.[5]
- NH+
4 + 2 [HgI
4]2− + 4 OH−
→ HgO · Hg(NH
2)I↓ + 7 I−
+ 3 H
2O
The brown precipitate is not fully characterized and may vary from HgO · Hg(NH
2)I to 3HgO · Hg(NH
3)
2I
2.[6]
References
- ↑ Lide, David R., ed (2009). CRC Handbook of Chemistry and Physics (90th ed.). Boca Raton, Florida: CRC Press. p. 4-82. ISBN 978-1-4200-9084-0.
- ↑ Template:VogelQualitative
- ↑ Wagenknecht, F.; Juza, R. (1963). "Potassium Triiodomercurate(II)". in Brauer, G.. Handbook of Preparative Inorganic Chemistry. 1 (2nd ed.). Academic Press. p. 1100. https://archive.org/details/Handbook_of_Preparative_Inorganic_Chemistry_1_2_Brauer/page/n1155/.
- ↑ Wagenknecht, F.; Juza, R. (1963). "Copper(I) Tetraiodomercurate(II)". in Brauer, G.. Handbook of Preparative Inorganic Chemistry. 1 (2nd ed.). Academic Press. p. 1100. https://archive.org/details/Handbook_of_Preparative_Inorganic_Chemistry_1_2_Brauer/page/n1155/.
- ↑ Template:VogelQualitative4th
- ↑ Template:VogelQualitative5th
External links
- IARC Monograph: "Mercury and Mercury Compounds"
- National Pollutant Inventory - Mercury and compounds fact sheet
 |

