Biology:Nirmatrelvir
 | |
| Clinical data | |
|---|---|
| Pronunciation | /nɜːrˈmætrəlˌvɪər/ nur-MA-trəl-veer or /ˌnɜːrməˈtrɛlvɪər/ NUR-mə-TREL-veer |
| Other names | PF-07321332, Bexovid |
| License data |
|
| Pregnancy category |
|
| Routes of administration | By mouth |
| ATC code |
|
| Legal status | |
| Legal status | |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEBI | |
| Chemical and physical data | |
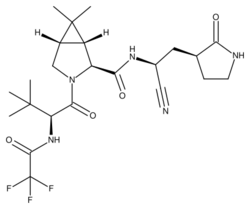
| Formula | C23H32F3N5O4 |
| Molar mass | 499.535 g·mol−1 |
| 3D model (JSmol) | |
| Melting point | 192.9 °C (379.2 °F) [3] |
| |
| |
Nirmatrelvir is an antiviral medication developed by Pfizer which acts as an orally active 3C-like protease inhibitor.[3][4][5][6][7] It is part of a nirmatrelvir/ritonavir combination used to treat COVID-19 and sold under the brand name Paxlovid.[8]
Development
Pharmaceutical
Coronaviral proteases cleave multiple sites in the viral polyprotein, usually after there are glutamine residues. Early work on related human rhinoviruses showed that the flexible glutamine side chain in inhibitors could be replaced by a rigid pyrrolidone.[9][10] These drugs had been further developed prior to the COVID-19 pandemic for other diseases including SARS.[11] The utility of targeting the 3CL protease in a real world setting was first demonstrated in 2018 when GC376 (a prodrug of GC373) was used to treat the previously 100% lethal cat coronavirus disease, feline infectious peritonitis, caused by feline coronavirus.[12] Nirmatrelvir and GC373 are both peptidomimetics, share the aforementioned pyrrolidone in P1 position and are competitive inhibitors. They use a nitrile and an aldehyde respectively to bind the catalytic cysteine.[13][14] Pfizer investigated two series of compounds, with nitrile and benzothiazol-2-yl ketone as the reactive group, respectively, and in the end settled on using nitrile.[15]
Nirmatrelvir was developed by modification of the earlier clinical candidate lufotrelvir,[16][full citation needed][17] which is also a covalent protease inhibitor but its active element is a phosphate prodrug of a hydroxyketone. Lufotrelvir needs to be administered intravenously limiting its use to a hospital setting. Stepwise modification of the tripeptide protein mimetic led to nirmatrelvir, which is suitable for oral administration.[3] Key changes include a reduction in the number of hydrogen bond donors, and the number of rotatable bonds by introducing a rigid bicyclic non-canonical amino acid (specifically, a "fused cyclopropyl ring with two methyl groups"[15]), which mimics the leucine residue found in earlier inhibitors. This residue had previously been used in the synthesis of boceprevir.[18] Tert-leucine (abbreviation: Tle) used in the P3 position of nirmatrelvir was identified first as optimal non-canonical amino acid in potential drug targeting SARS-CoV-2 3C-like protease using combinatorial chemistry (hybrid combinatorial substrate library technology).[19][20]
The leucine-like residue resulted in loss of a nearby contact with a glutamine on the 3C-like protease.[15] To compensate Pfizer tried adding methane sulfonamide, acetamide, and trifluoroacetamide and discovered that of the three, trifluoroacetamide resulted in superior oral bioavailability.[15]
Chemistry and pharmacology
Full details of the synthesis of nirmatrelvir were first published by scientists from Pfizer.[citation needed]
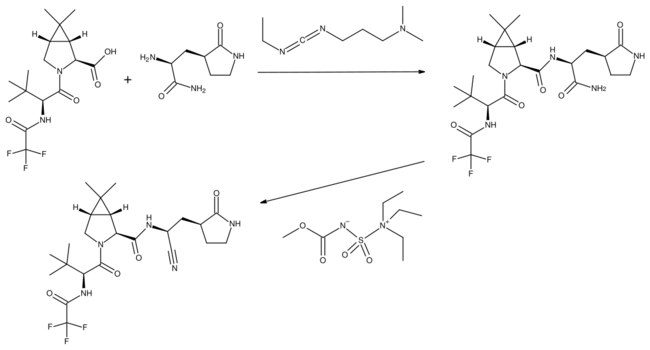
In the penultimate step a synthetic homochiral amino acid is coupled with a homochiral amino amide using the water-soluble carbodiimide EDCI as a coupling agent. The resulting intermediate is then treated with Burgess reagent, which dehydrates the amide group to the nitrile of the product.[3]
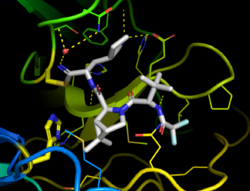
Nirmatrelvir is a covalent inhibitor, binding directly to the catalytic cysteine (Cys145) residue of the cysteine protease enzyme.[21]
In the co-packaged medication nirmatrelvir/ritonavir, ritonavir serves to slow the metabolism of nirmatrelvir via cytochrome enzyme inhibition, thereby increasing the circulating concentration of the main drug.[22] This effect is also used in HIV therapy, where ritonavir is used in combination with another protease inhibitor to similarly enhance their pharmacokinetics.[23]
Society and culture
Licensing
In November 2021, Pfizer signed a license agreement with the United Nations –backed Medicines Patent Pool to allow nirmatrelvir to be manufactured and sold in 95 countries.[24] Pfizer stated that the agreement will allow local medicine manufacturers to produce the pill "with the goal of facilitating greater access to the global population". The deal excludes several countries with major COVID-19 outbreaks including Brazil, China, Russia, Argentina, and Thailand.[25][26]
Usage for COVID-19 treatment
In December 2021, nirmatrelvir co-packaged with ritonavir (brand name Paxlovid) has been granted Emergency Use Authorization for the treatment of COVID-19 by the FDA.[27]
Research
The research that led to nirmatrelvir began on 16 March 2020, when Pfizer formally launched a project at its Cambridge, Massachusetts site to develop antiviral drugs for treating COVID-19.[15] On 22 July 2020, Pfizer chemists were able to synthesize nirmatrelvir for the first time, although the importance of that moment was not clear then because it was just one of 20 candidates synthesized that week.[15] On 1 September 2020, Pfizer completed a pharmacokinetic study in rats which suggested that nirmatrelvir could be administered orally.[15] The actual synthesis of the drug for laboratory research and for clinical trials was carried out at Pfizer's Groton, Connecticut site.[28]
In February 2021, Pfizer launched the company's first phase I trial of PF-07321332 (nirmatrelvir)[29] at its clinical research unit in New Haven, Connecticut.[28] According to Chemical & Engineering News, the drug went "from an idea to the first clinical test in a person in 12 months—a breathtaking pace at which to deliver a bespoke drug candidate."[15]
References
- ↑ "Updates to the Prescribing Medicines in Pregnancy database". 12 May 2022. https://www.tga.gov.au/updates-prescribing-medicines-pregnancy-database.
- ↑ "Notice: Nirmatrelvir (COVID-19) added to Prescription Drug List (PDL)". 17 January 2022. https://www.canada.ca/en/health-canada/services/drugs-health-products/drug-products/prescription-drug-list/notices-changes/amendment-nirmatrelvir.html.
- ↑ 3.0 3.1 3.2 3.3 "An oral SARS-CoV-2 Mpro inhibitor clinical candidate for the treatment of COVID-19". Science 374 (6575): 1586–1593. November 2021. doi:10.1126/science.abl4784. PMID 34726479. Bibcode: 2021Sci...374.1586O.
- ↑ "Antiviral treatment of COVID-19: An update". Turkish Journal of Medical Sciences 51 (SI-1): 3372–3390. August 2021. doi:10.3906/sag-2106-250. PMID 34391321.
- ↑ "Exploring the Binding Mechanism of PF-07321332 SARS-CoV-2 Protease Inhibitor through Molecular Dynamics and Binding Free Energy Simulations". International Journal of Molecular Sciences 22 (17): 9124. August 2021. doi:10.3390/ijms22179124. PMID 34502033.
- ↑ "Pfizer Announces Additional Phase 2/3 Study Results Confirming Robust Efficacy of Novel COVID-19 Oral Antiviral Treatment Candidate in Reducing Risk of Hospitalization or Death" (Press release). Pfizer. 14 December 2021. Retrieved 25 December 2021 – via Business Wire.
- ↑ "Considerations for the discovery and development of 3-chymotrypsin-like cysteine protease inhibitors targeting SARS-CoV-2 infection". Current Opinion in Virology 49: 36–40. August 2021. doi:10.1016/j.coviro.2021.04.006. PMID 34029993.
- ↑ "Paxlovid- nirmatrelvir and ritonavir kit". https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=7bdddfba-bd31-44cb-ba9e-23a4e17a4691.
- ↑ "Coronavirus Main Proteinase (3CLpro) Structure: Basis for Design of Anti-SARS Drugs". Science 300 (5626): 1763–1767. June 2003. doi:10.1126/science.1085658. PMID 12746549. Bibcode: 2003Sci...300.1763A.
- ↑ "Structure-based design, synthesis, and biological evaluation of irreversible human rhinovirus 3C protease inhibitors. 4. Incorporation of P1 lactam moieties as L-glutamine replacements". Journal of Medicinal Chemistry 42 (7): 1213–1224. April 1999. doi:10.1021/jm9805384. PMID 10197965.
- ↑ "An Overview of Severe Acute Respiratory Syndrome-Coronavirus (SARS-CoV) 3CL Protease Inhibitors: Peptidomimetics and Small Molecule Chemotherapy". Journal of Medicinal Chemistry 59 (14): 6595–6628. July 2016. doi:10.1021/acs.jmedchem.5b01461. PMID 26878082.
- ↑ "Efficacy of a 3C-like protease inhibitor in treating various forms of acquired feline infectious peritonitis". Journal of Feline Medicine and Surgery 20 (4): 378–392. April 2018. doi:10.1177/1098612X17729626. PMID 28901812.
- ↑ "Pfizer unveils its oral SARS-CoV-2 inhibitor". Chemical & Engineering News 99 (13): 7. 7 April 2021. doi:10.47287/cen-09913-scicon3.
- ↑ "Feline coronavirus drug inhibits the main protease of SARS-CoV-2 and blocks virus replication". Nature Communications 11 (1): 4282. August 2020. doi:10.1038/s41467-020-18096-2. PMID 32855413.
- ↑ 15.0 15.1 15.2 15.3 15.4 15.5 15.6 15.7 Halford, Bethany (14 January 2022). "How Pfizer scientists transformed an old drug lead into a COVID-19 antiviral: Behind the scenes of the medicinal chemistry campaign that led to the pill Paxlovid". Chemical & Engineering News 100 (3). https://cen.acs.org/pharmaceuticals/drug-discovery/How-Pfizer-scientists-transformed-an-old-drug-lead-into-a-COVID-19-antiviral/100/i3. Retrieved 14 January 2022.
- ↑ Clinical trial number NCT04535167 for "First-In-Human Study To Evaluate Safety, Tolerability, And Pharmacokinetics Following Single Ascending And Multiple Ascending Doses of PF-07304814 In Hospitalized Participants With COVID-19 " at ClinicalTrials.gov
- ↑ "Preclinical characterization of an intravenous coronavirus 3CL protease inhibitor for the potential treatment of COVID19". Nature Communications 12 (1): 6055. 2021. doi:10.1038/s41467-021-26239-2. PMID 34663813. Bibcode: 2021NatCo..12.6055B.
- ↑ "Challenges in modern drug discovery: a case study of boceprevir, an HCV protease inhibitor for the treatment of hepatitis C virus infection". Accounts of Chemical Research 41 (1): 50–59. January 2008. doi:10.1021/ar700109k. PMID 18193821.
- ↑ "Synthesis of a HyCoSuL peptide substrate library to dissect protease substrate specificity". Nature Protocols 12 (10): 2189–2214. October 2017. doi:10.1038/nprot.2017.091. PMID 28933778. https://zenodo.org/record/3629507.
- ↑ "SARS-CoV-2 M pro inhibitors and activity-based probes for patient-sample imaging". Nature Chemical Biology 17 (2): 222–228. October 2020. doi:10.1038/s41589-020-00689-z. PMID 33093684.
- ↑ "Supervised Molecular Dynamics (SuMD) Insights into the mechanism of action of SARS-CoV-2 main protease inhibitor PF-07321332". Journal of Enzyme Inhibition and Medicinal Chemistry 36 (1): 1646–1650. December 2021. doi:10.1080/14756366.2021.1954919. PMID 34289752.
- ↑ "What is Australia's potential new COVID treatment?". 19 October 2021. https://www1.racgp.org.au/newsgp/clinical/what-is-australia-s-potential-new-covid-treatment.
- ↑ "Ritonavir-boosted protease inhibitors in HIV therapy". Annals of Medicine 43 (5): 375–388. April 2011. doi:10.3109/07853890.2011.572905. PMID 21501034.
- ↑ "Pfizer and The Medicines Patent Pool (MPP) Sign Licensing Agreement for COVID-19 Oral Antiviral Treatment Candidate to Expand Access in Low- and Middle-Income Countries" (Press release). Pfizer. 16 November 2021. Retrieved 17 November 2021 – via Business Wire.
- ↑ "Covid-19: Pfizer to allow developing nations to make its treatment pill". BBC News. 16 November 2021. https://www.bbc.com/news/world-us-canada-59310582.
- ↑ "Pfizer Will Allow Its Covid Pill to Be Made and Sold Cheaply in Poor Countries". 16 November 2021. https://www.nytimes.com/2021/11/16/health/covid-pill-pfizer.html.
- ↑ "FDA grants EUA for Pfizer’s Covid-19 oral antiviral Paxlovid". 23 December 2021. https://www.pharmaceutical-technology.com/news/fda-eua-pfizer-paxlovid/.
- ↑ 28.0 28.1 Green, Rick (23 December 2021). "Pfizer scientists in Groton played a critical role in development of new COVID-19 pill". The Hartford Courant. https://www.courant.com/coronavirus/hc-news-coronavirus-pfizer-paxlovid-connecticut-groton-researchers-20211223-hsurttz4lrbt7o4nijvoq5qxui-story.html.
- ↑ Study Of PF-07321332 In Healthy Participants. clinicaltrials.gov. 18 October 2021. https://clinicaltrials.gov/ct2/show/NCT04756531.
External links
- "Nirmatrelvir". Drug Information Portal. U.S. National Library of Medicine. https://druginfo.nlm.nih.gov/drugportal/name/nirmatrelvir.