Chemistry:Agroclavine
From HandWiki
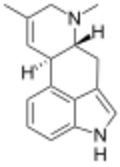
| |
| Names | |
|---|---|
| IUPAC name
6,8-Dimethyl-8,9-didehydroergoline
| |
| Systematic IUPAC name
(6aR,10aR)-7,9-Dimethyl-4,6,6a,7,8,10a-hexahydroindolo[4,3-fg]quinoline | |
| Other names
8,9-Didehydro-6,8-dimethylergoline
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
PubChem CID
|
|
| UNII | |
| |
| |
| Properties | |
| C16H18N2 | |
| Molar mass | 238.334 g·mol−1 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Tracking categories (test):
Agroclavine belongs to the group of ergot alkaloids, which also includes ergotamine.[1] Historically, the main use of agroclavine was in the synthesis of ergot-based drugs; agroclavine can be oxidized to elymoclavine, which then undergoes further processing.[2]
References
- ↑ Bhattacharji, S.; Birch, A. J.; Brack, A.; Hofmann, A.; Kobel, H.; Smith, D. C. C.; Smith, Herchel; Winter J. (1962). "Biosynthesis. XXVII. The biosynthesis of ergot alkaloids". Journal of the Chemical Society: 421–425. doi:10.1039/jr9620000421.
- ↑ The Alkaloids: Chemistry and Biology. Gulf Professional Publishing. 2002. pp. 6–. ISBN 978-0-12-469558-0. https://books.google.com/books?id=dush_I9WbWsC&pg=PA6.
 |

