Chemistry:Psilocybin
Psilocybin, also known as 4-phosphoryloxy-N,N-dimethyltryptamine (4-PO-DMT),[lower-alpha 1] is a naturally occurring tryptamine alkaloid and investigational drug found in more than 200 species of mushrooms, with hallucinogenic and serotonergic effects.[2][3] Effects include euphoria, changes in perception, a distorted sense of time (via brain desynchronization),[4] and perceived spiritual experiences. It can also cause adverse reactions such as nausea and panic attacks.
Psilocybin is a prodrug of psilocin.[2] That is, the compound itself is biologically inactive but quickly converted by the body to psilocin.[2] Psilocybin is transformed into psilocin by dephosphorylation mediated via phosphatase enzymes.[5][2] Psilocin is chemically related to the neurotransmitter serotonin and acts as a non-selective agonist of the serotonin receptors.[2] Activation of one serotonin receptor, the serotonin 5-HT2A receptor, is specifically responsible for the hallucinogenic effects of psilocin and other serotonergic psychedelics.[2] Psilocybin is usually taken orally.[2] By this route, its onset is about 20 to 50 minutes, peak effects occur after around 60 to 90 minutes, and its duration is about 4 to 6 hours.[6][7][2][8]
Psilocybin mushrooms were used ritualistically in pre-Columbian Mexico, but claims of their widespread ancient use are largely exaggerated and shaped by modern idealization and ideology.[9] In 1958, the Swiss chemist Albert Hofmann isolated psilocybin and psilocin from the mushroom Psilocybe mexicana. His employer, Sandoz, marketed and sold pure psilocybin to physicians and clinicians worldwide for use in psychedelic therapy. Increasingly restrictive drug laws of the 1960s and the 1970s curbed scientific research into the effects of psilocybin and other hallucinogens, but its popularity as an entheogen grew in the next decade, owing largely to the increased availability of information on how to cultivate psilocybin mushrooms.
Possession of psilocybin-containing mushrooms has been outlawed in most countries, and psilocybin has been classified as a Schedule I controlled substance under the 1971 United Nations Convention on Psychotropic Substances. Psilocybin is being studied as a possible medicine in the treatment of psychiatric disorders such as depression, substance use disorders, obsessive–compulsive disorder, and other conditions such as cluster headaches.[10] Psilocybin was approved for treatment-resistant depression in Australia in 2023.[11][12] It is in late-stage clinical trials in the United States for treatment-resistant depression.[13][14][15] Especially at higher doses and combined with psychological support, psilocybin can produce rapid and sometimes lasting antidepressant effects that generally outperform placebo but show only modest advantages over conventional SSRIs; evidence quality is generally low and trial bias is common.
Uses
Psilocybin is used recreationally, spiritually (as an entheogen), and medically.[16] It is primarily taken orally, but other routes of administration, such as intravenous injection, are sometimes employed by licensed medical researchers using pharmaceutical-grade psilocybin powder designed for injection. Injection should never be attempted by unlicensed people.[17]
Medical
In 2023, the Therapeutic Goods Administration (TGA) approved psilocybin for treatment of treatment-resistant depression in Australia.[18][19][20] It is also under development for the treatment of depression and for various other indications elsewhere, such as the United States and Europe, but has not yet been approved in other countries (see below).[21][10][15]
Dosing
Psilocybin is used as a psychedelic at doses of 5 to 40 mg orally.[8][22] Low doses are 5 to 10 mg, an intermediate or "good effect" dose is 20 mg, and high or ego-dissolution doses are 30 to 40 mg.[8][22] Psilocybin's effects can be subjectively perceived at a dose as low as 3 mg per 70 kg body weight.[22][23] Microdosing involves the use of subthreshold psilocybin doses of less than 2.5 mg.[8][22]
When psilocybin is used in the form of psilocybin-containing mushrooms, microdoses are 0.1 g to 0.3 g and psychedelic doses are 1.0 g to 3.5–5.0 g in the case of dried mushrooms.[24][25][17] The preceding 1.0 to 5.0 g range corresponds to psilocybin doses of about 10 to 50 mg.[17] Psilocybin-containing mushrooms vary in their psilocybin and psilocin content, but are typically around 1% of the dried weight of the mushrooms (in terms of total or combined psilocybin and psilocin content).[25][26][27][17][5][28][29][30] Psilocin is about 1.4 times as potent as psilocybin because of the two compounds' difference in molecular weight.[27][31][32]
Available forms
Psilocybin is most commonly consumed in the form of psilocybin-containing mushrooms, such as Psilocybe species like Psilocybe cubensis. It may also be prepared synthetically, but outside of research settings it is not typically used in this form. Regardless of form, psilocybin is usually taken orally. The psilocybin present in certain species of mushrooms can be ingested in several ways: by consuming fresh or dried fruit bodies, by preparing an herbal tea, or by combining with other foods to mask the bitter taste.[33] In rare cases people have intravenously injected mushroom extracts, with serious medical complications such as systemic mycological infection and hospitalization.[34][35][36][37] Another form of psilocybin (as well as of related psychedelics like 4-AcO-DMT) is mushroom edibles such as chocolate bars and gummies, which may be purchased at psychedelic mushroom stores.
Effects

Psilocybin produces a variety of psychological, perceptual, interpersonal, and physical effects.[8]
Psychological and perceptual effects
After ingesting psilocybin, the user may experience a wide range of emotional effects, which can include disorientation, lethargy, giddiness, euphoria, joy, and depression. In one study, 31% of volunteers given a high dose reported feelings of significant fear and 17% experienced transient paranoia.[34] In studies at Johns Hopkins, among those given a moderate dose (but enough to "give a high probability of a profound and beneficial experience"), negative experiences were rare, whereas one-third of those given a high dose experienced anxiety or paranoia.[38][39] Low doses can induce hallucinatory effects. Closed-eye hallucinations may occur, where the affected person sees multicolored geometric shapes and vivid imaginative sequences.[23] Some people report synesthesia, such as tactile sensations when viewing colors.[40]: 175 At higher doses, psilocybin can lead to "intensification of affective responses, enhanced ability for introspection, regression to primitive and childlike thinking, and activation of vivid memory traces with pronounced emotional undertones".[41] Open-eye visual hallucinations are common and may be very detailed, although rarely confused with reality.[23]
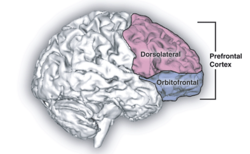
Psilocybin is known to strongly affect the subjective experience of the passage of time.[42][4] Users often feel as if time is slowed down, resulting in the perception that "minutes appear to be hours" or "time is standing still".[43] Studies have demonstrated that psilocybin significantly impairs subjects' ability to gauge time intervals longer than 2.5 seconds, impairs their ability to synchronize to inter-beat intervals longer than 2 seconds, and reduces their preferred tapping rate.[43][44] These results are consistent with the drug's role in affecting prefrontal cortex activity[45] and the role that the prefrontal cortex plays in time perception,[46] but the neurochemical basis of psilocybin's effects on perception of time is not known with certainty.[47]
Users having a pleasant experience can feel a sense of connection to others, nature, and the universe; other perceptions and emotions are also often intensified. Users having an unpleasant experience (a "bad trip") describe a reaction accompanied by fear, other unpleasant feelings, and occasionally by dangerous behavior. The term "bad trip" is generally used to describe a reaction characterized primarily by fear or other unpleasant emotions, not just a transitory experience of such feelings. A variety of factors may contribute to a bad trip, including "tripping" during an emotional or physical low or in a non-supportive environment (see: set and setting). Ingesting psilocybin in combination with other drugs, including alcohol, can also increase the likelihood of a bad trip.[34][48] Other than the duration of the experience, the effects of psilocybin are similar to comparable doses of lysergic acid diethylamide (LSD) or mescaline. But in the Psychedelics Encyclopedia, author Peter Stafford writes: "The psilocybin experience seems to be warmer, not as forceful and less isolating. It tends to build connections between people, who are generally much more in communication than when they use LSD."[49]: 273
Set and setting and moderating factors
The effects of psilocybin are highly variable and depend on the mindset and environment in which the user has the experience, factors commonly called set and setting. In the early 1960s, Timothy Leary and his Harvard colleagues investigated the role of set and setting in psilocybin's effects. They administered the drug to 175 volunteers (from various backgrounds) in an environment intended to be similar to a comfortable living room. 98 of the subjects were given questionnaires to assess their experiences and the contribution of background and situational factors. Those who had prior experience with psilocybin reported more pleasant experiences than those for whom the drug was novel. Group size, dose, preparation, and expectancy were important determinants of the drug response. In general, those in groups of more than eight felt that the groups were less supportive and their experiences less pleasant. Conversely, smaller groups (fewer than six) were seen as more supportive and reported more positive reactions to the drug in those groups. Leary and colleagues proposed that psilocybin heightens suggestibility, making a user more receptive to interpersonal interactions and environmental stimuli.[50] These findings were affirmed in a later review by Jos ten Berge (1999), who concluded that dose, set, and setting are fundamental factors in determining the outcome of experiments that tested the effects of psychedelic drugs on artists' creativity.[51]
Further studies demonstrate that supportive settings significantly reduce the likelihood of adverse reactions, including panic, paranoia, or psychological distress. Positive therapeutic outcomes are strongly correlated with the participant's trust in the environment and the facilitators.[52][53]
Theory of mind network and default mode network
Psychedelics, including psilocybin, have been shown to affect different clusters of brain regions known as the "theory of mind network" (ToMN) and the default mode network (DMN).[54] The ToMN involves making inferences and understanding social situations based on patterns,[55] whereas the DMN relates more to introspection and one's sense of self.[54] The DMN, in particular, is related to increased rumination and worsening self-image in patients with major depressive disorder (MDD).[56] In studies done with single use psilocybin, areas of the DMN showed decreased functional connectivity (communication between areas of the brain). This provides functional insight into the work of psilocybin in increasing one's sense of connection to one's surroundings, as the areas of the brain involved in introspection decrease in functionality under the effects of the drug.[57] Conversely, areas of the brain involved in the ToMN showed increased activity and functional activation in response to psychedelics. These results were not unique to psilocybin and there was no significant difference in brain activation found in similar trials of mescaline and LSD. Information and studies into the DMN and ToMN are relatively sparse and their connections to other psychiatric illnesses and the use of psychedelics is still largely unknown.[54]
Group perceptions
Through further anthropological studies regarding "personal insights"[58] and the psychosocial effects of psilocybin, it can be seen in many traditional societies that powerful mind-active substances such as psilocybin are regularly "consumed ritually for therapeutic purposes or for transcending normal, everyday reality".[59] Positive effects that psilocybin has on individuals can be observed by taking on an anthropological approach and moving away from the Western biomedical view; this is aided by the studies done by Leary.[60] Within certain traditional societies, where the use of psilocybin is frequent for shamanic healing rituals, group collectives praise their guide, healer and shaman for helping alleviate their pains, aches and hurt. They do this through a group ritual practice where the group, or just the guide, ingests psilocybin to help extract any "toxic psychic residues or sorcerous implants"[59] found in one's body.
Group therapies using "classic" psychedelics are becoming more commonly used in the Western world in clinical practice.[61] This is speculated to grow, provided the evidence remains indicative of their safety and efficacy.[62] In social sense, the group is shaped by their experiences surrounding psilocybin and how they view the fungus collectively. As mentioned in the anthropology article,[59] the group partakes in a "journey" together, thus adding to the spiritual, social body where roles, hierarchies and gender are subjectively understood.[59]
Cultural significance and "mystical" experiences

Psilocybin mushrooms have been and continue to be used in Indigenous American cultures in religious, divinatory, or spiritual contexts. Reflecting the meaning of the word entheogen ("the god within"), the mushrooms are revered as powerful spiritual sacraments that provide access to sacred worlds. Typically used in small group community settings, they enhance group cohesion and reaffirm traditional values.[63] Terence McKenna documented the worldwide practices of psilocybin mushroom usage as part of a cultural ethos relating to the Earth and mysteries of nature, and suggested that mushrooms enhanced self-awareness and a sense of contact with a "Transcendent Other"—reflecting a deeper understanding of our connectedness with nature.[64]
Psychedelic drugs can induce states of consciousness that have lasting personal meaning and spiritual significance in religious or spiritually inclined people; these states are called mystical experiences. Some scholars have proposed that many of the qualities of a drug-induced mystical experience are indistinguishable from mystical experiences achieved through non-drug techniques such as meditation or holotropic breathwork.[65][66] In the 1960s, Walter Pahnke and colleagues systematically evaluated mystical experiences (which they called "mystical consciousness") by categorizing their common features. According to Pahnke, these categories "describe the core of a universal psychological experience, free from culturally determined philosophical or theological interpretations", and allow researchers to assess mystical experiences on a qualitative, numerical scale.[67]
In the 1962 Marsh Chapel Experiment, run by Pahnke at the Harvard Divinity School under Leary's supervision,[68] almost all the graduate degree divinity student volunteers who received psilocybin reported profound religious experiences.[69] One of the participants was religious scholar Huston Smith, author of several textbooks on comparative religion; he called his experience "the most powerful cosmic homecoming I have ever experienced."[70] In a 25-year followup to the experiment, all the subjects given psilocybin said their experience had elements of "a genuine mystical nature and characterized it as one of the high points of their spiritual life".[71]: 13 Psychedelic researcher Rick Doblin considered the study partially flawed due to incorrect implementation of the double-blind procedure and several imprecise questions in the mystical experience questionnaire. Nevertheless, he said that the study cast "considerable doubt on the assertion that mystical experiences catalyzed by drugs are in any way inferior to non-drug mystical experiences in both their immediate content and long-term effects".[71]: 24 Psychiatrist William A. Richards echoed this sentiment, writing in a 2007 review, "[psychedelic] mushroom use may constitute one technology for evoking revelatory experiences that are similar, if not identical, to those that occur through so-called spontaneous alterations of brain chemistry."[72]
A group of researchers from Johns Hopkins School of Medicine led by Roland Griffiths conducted a study to assess the immediate and long-term psychological effects of the psilocybin experience, using a modified version of the mystical experience questionnaire and a rigorous double-blind procedure.[73] When asked in an interview about the similarity of his work to Leary's, Griffiths explained the difference: "We are conducting rigorous, systematic research with psilocybin under carefully monitored conditions, a route which Dr. Leary abandoned in the early 1960s."[74] Experts have praised the National Institute of Drug Abuse-funded study, published in 2006, for the soundness of its experimental design.[lower-alpha 2] In the experiment, 36 volunteers with no experience with hallucinogens were given psilocybin and methylphenidate (Ritalin) in separate sessions; the methylphenidate sessions served as a control and psychoactive placebo. The degree of mystical experience was measured using a questionnaire developed by Ralph W. Hood;[75] 61% of subjects reported a "complete mystical experience" after their psilocybin session, while only 13% reported such an outcome after their experience with methylphenidate. Two months after taking psilocybin, 79% of the participants reported moderately to greatly increased life satisfaction and sense of well-being. About 36% of participants also had a strong to extreme "experience of fear" or dysphoria (i.e., a "bad trip") at some point during the psilocybin session (which was not reported by any subject during the methylphenidate session); about one-third of these (13% of the total) reported that this dysphoria dominated the entire session. These negative effects were reported to be easily managed by the researchers and did not have a lasting negative effect on the subject's sense of well-being.[76]
A follow-up study 14 months later confirmed that participants continued to attribute deep personal meaning to the experience. Almost a third of the subjects reported that the experience was the single most meaningful or spiritually significant event of their lives, and over two-thirds reported it was among their five most spiritually significant events. About two-thirds said the experience increased their sense of well-being or life satisfaction.[69] Even after 14 months, those who reported mystical experiences scored on average 4 percentage points higher on the personality trait of Openness/Intellect; personality traits are normally stable across the lifespan for adults. Likewise, in a 2010 web-based questionnaire study designed to investigate user perceptions of the benefits and harms of hallucinogenic drug use, 60% of the 503 psilocybin users reported that their use of psilocybin had a long-term positive impact on their sense of well-being.[34][77]
Physical effects
Common responses include pupil dilation (93%); changes in heart rate (100%), including increases (56%), decreases (13%), and variable responses (31%); changes in blood pressure (84%), including hypotension (34%), hypertension (28%), and general instability (22%); changes in stretch reflex (86%), including increases (80%) and decreases (6%); nausea (44%); tremor (25%); and dysmetria (16%) (inability to properly direct or limit motions).[78][lower-alpha 3] Psilocybin's sympathomimetic or cardiovascular effects, including increased heart rate and blood pressure, are usually mild.[16][78] On average, peak heart rate is increased by 5 bpm, peak systolic blood pressure by 10 to 15 mm Hg, and peak diastolic blood pressure by 5 to 10 mm Hg.[16][78] But temporary increases in blood pressure can be a risk factor for users with preexisting hypertension.[23] Psilocybin's somatic effects have been corroborated by several early clinical studies.[80] A 2005 magazine survey of clubgoers in the UK found that over a quarter of those who had used psilocybin mushrooms in the preceding year experienced nausea or vomiting, although this was caused by the mushroom rather than psilocybin itself.[34] In one study, administration of gradually increasing doses of psilocybin daily for 21 days had no measurable effect on electrolyte levels, blood sugar levels, or liver toxicity tests.[78]
Onset and duration
The onset of action of psilocybin taken orally is 0.5 to 0.8 hours (30–50 minutes) on average, with a range of 0.1 to 1.5 hours (5–90 minutes).[8][6] Peak psychoactive effects occur at about 1.0 to 2.2 hours (60–130 minutes).[6][8] The time to offset of psilocybin orally is about 6 to 7 hours on average.[81] The duration of action of psilocybin is about 4 to 6 hours (range 3–12 hours) orally.[6][8][7] A small dose of 1 mg by intravenous injection had a duration of 15 to 30 minutes.[78][82] In another study, 2 mg psilocybin by intravenous injection given over 60 seconds had an immediate onset, reached a sustained peak after 4 minutes, and subsided completely after 45 to 60 minutes.[83][84]
Contraindications
Contraindications of psilocybin are mostly psychiatric conditions that increase the risk of psychological distress, including the rare adverse effect of psychosis during or after the psychedelic experience.[6][85] These conditions may include history of psychosis, schizophrenia, bipolar disorder, or borderline personality disorder.[6][86] Further research may provide more safety information about the use of psilocybin in people with such conditions.[6] It is notable in this regard that psilocybin and other psychedelics are being studied for the potential treatment of all the preceding conditions.[87][88][89][90][91][92]
Psilocybin is also considered to be contraindicated in women who are pregnant or breastfeeding due to insufficient research in this population.[6] There are transient increases in heart rate and blood pressure with psilocybin, and hence uncontrolled cardiovascular conditions are a relative contraindication for psilocybin.[6] Serotonin 5-HT2A receptor antagonists such as atypical antipsychotics and certain antidepressants may block psilocybin's hallucinogenic effects and hence may be considered contraindicated in this sense.[93][94] Monoamine oxidase inhibitors (MAOIs) may potentiate psilocybin's effects and augment its risks.[93]
Adverse effects
Most of the comparatively few fatal incidents associated with psychedelic mushroom usage involve the simultaneous use of other drugs, especially alcohol. A common adverse effect resulting from psilocybin mushroom use involves "bad trips" or panic reactions, in which people become anxious, confused, agitated, or disoriented.[95] Accidents, self-injury, or suicide attempts can result from serious cases of acute psychotic episodes.[34] No studies have linked psilocybin with birth defects,[96] but it is recommended that pregnant women avoid its usage.[97]
Psychiatric adverse effects
Panic reactions can occur after consumption of psilocybin-containing mushrooms, especially if the ingestion is accidental or otherwise unexpected. Reactions characterized by violent behavior, suicidal thoughts,[98] schizophrenia-like psychosis,[99][100] and convulsions[101] have been reported in the literature. A 2005 survey conducted in the United Kingdom found that almost a quarter of those who had used psilocybin mushrooms in the past year had experienced a panic attack.[34] Less frequently reported adverse effects include paranoia, confusion, prolonged derealization (disconnection from reality), and mania.[77] Psilocybin usage can temporarily induce a state of depersonalization disorder.[102] Usage by those with schizophrenia can induce acute psychotic states requiring hospitalization.[103]
The similarity of psilocybin-induced symptoms to those of schizophrenia has made the drug a useful research tool in behavioral and neuroimaging studies of schizophrenia.[104][105][106] In both cases, psychotic symptoms are thought to arise from a "deficient gating of sensory and cognitive information" in the brain that leads to "cognitive fragmentation and psychosis".[105] Flashbacks (spontaneous recurrences of a previous psilocybin experience) can occur long after psilocybin use. Hallucinogen persisting perception disorder (HPPD) is characterized by a continual presence of visual disturbances similar to those generated by psychedelic substances. Neither flashbacks nor HPPD are commonly associated with psilocybin usage,[34] and correlations between HPPD and psychedelics are further obscured by polydrug use and other variables.[107]
Tolerance and dependence
Tolerance to psilocybin builds and dissipates quickly; ingesting it more than about once a week can lead to diminished effects. Tolerance dissipates after a few days, so doses can be spaced several days apart to avoid the effect.[109] A cross-tolerance can develop between psilocybin and LSD,[110] and between psilocybin and phenethylamines such as mescaline and DOM.[111]
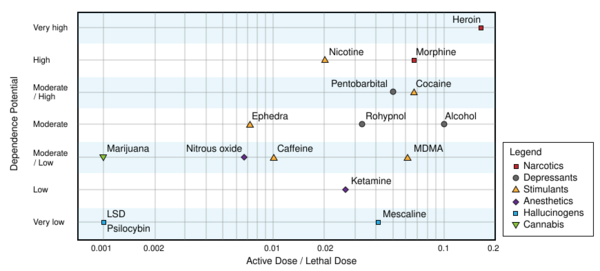
Repeated use of psilocybin does not lead to physical dependence.[78] A 2008 study concluded that, based on U.S. data from 2000 to 2002, adolescent-onset (defined here as ages 11–17) usage of hallucinogenic drugs (including psilocybin) did not increase the risk of drug dependence in adulthood; this was in contrast to adolescent usage of cannabis, cocaine, inhalants, anxiolytic medicines, and stimulants, all of which were associated with "an excess risk of developing clinical features associated with drug dependence".[112] Likewise, a 2010 Dutch study ranked the relative harm of psilocybin mushrooms compared to a selection of 19 recreational drugs, including alcohol, cannabis, cocaine, ecstasy, heroin, and tobacco. Psilocybin mushrooms were ranked as the illicit drug with the lowest harm,[113] corroborating conclusions reached earlier by expert groups in the United Kingdom.[114]
Long-term effects
A potential risk of frequent repeated use of psilocybin and other psychedelics is cardiac fibrosis and valvulopathy caused by serotonin 5-HT2B receptor activation.[115][116] But single high doses or widely spaced doses (e.g., months apart) are thought to be safe, and concerns about cardiac toxicity apply more to chronic psychedelic microdosing or very frequent intermittent use (e.g., weekly).[115][116]
Overdose
Psilocybin has low toxicity, meaning that it has a low risk of inducing life-threatening events like breathing or heart problems.[117][95] Research shows that health risks may develop with use of psilocybin. Nonetheless, hospitalizations from it are rare, and overdoses are generally mild and self-limiting.[117][95] The lethal dose of psilocybin in humans is unknown, but has been estimated to be approximately 200 times a typical recreational dose.[117]
A review of the management of psychedelic overdoses suggested that psilocybin-related overdose management should prioritize managing the immediate adverse effects, such as anxiety and paranoia, rather than specific pharmacological interventions, as psilocybin's physiological toxicity tends to be rather limited.[118] One analysis of people hospitalized for psilocybin poisoning found high urine concentrations of phenethylamine (PEA), suggesting that PEA might contribute to the effects of psilocybin poisoning.[118]
Despite acting as non-selective serotonin receptor agonists, psilocybin and other major serotonergic psychedelics like lysergic acid diethylamide (LSD) do not cause serotonin syndrome even in the context of extreme overdose.[119][117][120] This is thought to be because they act as partial agonists of serotonin receptors like the serotonin 5-HT2A receptor, in contrast to serotonin itself, which is a full agonist.[119][120]
In rats, the median lethal dose (LD50) of psilocybin when administered orally is 280 mg/kg, approximately 1.5 times that of caffeine. The lethal dose of psilocybin when administered intravenously in mice is 285 mg/kg, in rats is 280 mg/kg, and in rabbits is 12.5 mg/kg.[121][122] Psilocybin comprises approximately 1% of the weight of Psilocybe cubensis mushrooms, and so nearly 1.7 kilograms (3.7 lb) of dried mushrooms, or 17 kilograms (37 lb) of fresh mushrooms, would be required for a 60-kilogram (130 lb) person to reach the 280 mg/kg LD50 value of rats.[34] Based on the results of animal studies and limited human case reports, the human lethal dose of psilocybin has been extrapolated to be 2,000 to 6,000 mg, which is around 1,000 times greater than its effective dose of 6 mg and 200 times the typical recreational dose of 10 to 30 mg.[123][117] The Registry of Toxic Effects of Chemical Substances assigns psilocybin a relatively high therapeutic index of 641 (higher values correspond to a better safety profile); for comparison, the therapeutic indices of aspirin and nicotine are 199 and 21, respectively.[124] The lethal dose from psilocybin toxicity alone is unknown, and has rarely been documented—as of 2011[update], only two cases attributed to overdosing on hallucinogenic mushrooms (without concurrent use of other drugs) have been reported in the scientific literature, and those may involve factors other than psilocybin.[34][lower-alpha 4]
Interactions
Serotonin 5-HT2A receptor antagonists can block the hallucinogenic effects of serotonergic psychedelics like psilocybin.[93][127] Numerous drugs act as serotonin 5-HT2A receptor antagonists, including antidepressants like trazodone and mirtazapine, antipsychotics like quetiapine, olanzapine, and risperidone, and other agents like ketanserin, pimavanserin, cyproheptadine, and pizotifen.[93][94] Such drugs are sometimes called "trip killers" because they can prevent or abort psychedelics' hallucinogenic effects.[128][94][129] Serotonin 5-HT2A receptor antagonists that have been specifically shown in clinical studies to diminish or abolish psilocybin's effects include ketanserin, risperidone, and chlorpromazine.[93][127]
The serotonin 5-HT1A receptor partial agonist buspirone has been found to markedly reduce psilocybin's hallucinogenic effects in humans.[93][127][130][131] Conversely, the serotonin 5-HT1A receptor antagonist pindolol has been found to potentiate the hallucinogenic effects of the related psychedelic dimethyltryptamine (DMT) by 2- to 3-fold in humans.[131][132] Selective serotonin reuptake inhibitors (SSRIs) may modify psilocybin's effects.[93][127][133] One clinical trial found that psilocybin's hallucinogenic and "good drug" effects were not modified by the SSRI escitalopram, but that its "bad drug effects" such as anxiety, as well as ego dissolution, were reduced, among other changes.[127][93][133]
Benzodiazepines such as diazepam, alprazolam, clonazepam, and lorazepam, as well as alcohol, which act as GABAA receptor positive allosteric modulators, have been limitedly studied in combination with psilocybin and other psychedelics and are not known to directly interact with them.[127][93] But these GABAergic drugs produce effects such as anxiolysis, sedation, and amnesia, and may therefore diminish or otherwise oppose psychedelics' effects.[93][128][94][129][134] Because of this, recreational users often use benzodiazepines and alcohol as "trip killers" to manage difficult hallucinogenic experiences with psychedelics, such as experiences with prominent anxiety.[128][94][129] This strategy's safety is not entirely clear and might have risks,[128][127][94][129] but benzodiazepines have been used to manage psychedelics' adverse psychological effects in clinical studies and in Emergency Rooms.[127][135][136][137][138] A clinical trial of psilocybin and midazolam coadministration found that midazolam clouded psilocybin's effects and impaired memory of the experience.[139][140] Benzodiazepines might interfere with the therapeutic effects of psychedelics like psilocybin, such as sustained antidepressant effects.[141][142]
Psilocin, the active form of psilocybin, is a substrate of the monoamine oxidase (MAO) enzyme MAO-A.[143][81][27] The exact extent to which psilocin (and by extension psilocybin) is metabolized by MAO-A is not fully clear, but has ranged from 4% to 33% in different studies based on metabolite excretion.[143][81][27] Circulating levels of psilocin's deaminated metabolite are far higher than those of free unmetabolized psilocin with psilocybin administration.[21][82] Combination of MAO-substrate psychedelics with monoamine oxidase inhibitors (MAOIs) can result in overdose and toxicity.[93] Examples of MAOIs that may potentiate psychedelics behaving as MAO-A substrates, such as psilocin, include phenelzine, tranylcypromine, isocarboxazid, and moclobemide, as well as harmala alkaloids like harmine and harmaline and chronic tobacco smoking.[93][144] An early clinical study of psilocybin in combination with short-term tranylcypromine pretreatment found that tranylcypromine marginally potentiated psilocybin's peripheral effects, including pressor effects and mydriasis, but overall did not significantly modify its psychoactive and hallucinogenic effects, although some of its emotional effects were said to be reduced and some of its perceptual effects were said to be amplified.[16][145][146]
Psilocin may be metabolized to a minor extent by the cytochrome P450 (CYP450) enzymes CYP2D6 and/or CYP3A4 and appears unlikely to be metabolized by other CYP450 enzymes.[143][16] The role of CYP450 enzymes in psilocin's metabolism seems to be small, and so considerable drug interactions with CYP450 inhibitors and/or inducers may not be expected.[143][16] Psilocin's major metabolic pathway is glucuronidation by UDP-glucuronosyltransferase enzymes including UGT1A10 and UGT1A9.[127] Diclofenac and probenecid are inhibitors of these enzymes that theoretically might inhibit the metabolism of and thereby potentiate psilocybin's effects,[127] but no clinical research or evidence on this possible interaction exists.[127] Few other drugs are known to influence UGT1A10 or UGT1A9 function.[127]
Pharmacology
Pharmacodynamics
Psilocybin is a serotonergic psychedelic that acts as a prodrug of psilocin, the active form of the drug.[8][21] Psilocin is a close analogue of the monoamine neurotransmitter serotonin and, like serotonin, acts as a non-selective agonist of the serotonin receptors, including behaving as a partial agonist of the serotonin 5-HT2A receptor.[8][21][27] It shows high affinity for most of the serotonin receptors, with the notable exception of the serotonin 5-HT3 receptor.[8][21][27] Psilocin's affinity for the serotonin 5-HT2A receptor is 15-fold higher in humans than in rats due to species differences.[27][147] In addition to interacting with the serotonin receptors, psilocin is a partial serotonin releasing agent with lower potency.[148][149] Unlike certain other psychedelics such as LSD, it appears to show little affinity for many other targets, such as dopamine receptors.[2][8][16][150][151][152] Psilocin is an agonist of the mouse and rat but not human trace amine-associated receptor 1 (TAAR1).[153][150][154]
Psilocybin's and psilocin's psychedelic effects are mediated specifically by agonism of the serotonin 5-HT2A receptor.[8][21] Selective serotonin 5-HT2A receptor antagonists like volinanserin block the head-twitch response (HTR), a behavioral proxy of psychedelic-like effects, induced by psilocybin in rodents, and the HTR is similarly absent in serotonin 5-HT2A receptor knockout mice.[21][27][155][154] There is a significant relationship between psilocybin's hallucinogenic effects and serotonin 5-HT2A receptor occupancy in humans.[8][111][156] Psilocybin's psychedelic effects can be blocked by serotonin 5-HT2A receptor antagonists like ketanserin and risperidone in humans.[157][8][21][111][99] Activation of serotonin 5-HT2A receptors in layer V of the medial prefrontal cortex (mPFC) and consequent glutamate release in this area has been especially implicated in the hallucinogenic effects of psilocybin and other serotonergic psychedelics.[158][159][160][161][162] In addition, region-dependent alterations in brain glutamate levels may be related to the experience of ego dissolution.[163] The cryo-EM structures of the serotonin 5-HT2A receptor with psilocin, as well as with various other psychedelics and serotonin 5-HT2A receptor agonists, have been solved and published by Bryan L. Roth and colleagues.[164][165]
Although serotonin 5-HT2A receptor agonism mediates the hallucinogenic effects of psilocybin and psilocin, activation of other serotonin receptors also appears to contribute to these compounds' psychoactive and behavioral effects.[111][21][27][166][167][168] Serotonin 5-HT1A receptor activation seems to inhibit the hallucinogenic effects of psilocybin and other psychedelics.[93][130][131][132] Some of psilocybin's non-hallucinogenic behavioral effects in animals can be reversed by antagonists of the serotonin 5-HT1A, 5-HT2B, and 5-HT2C receptors.[21][27] Psilocybin produces profoundly decreased locomotor and investigatory behavior in rodents, and this appears to be dependent on serotonin 5-HT1A receptor activation but not on activation of the serotonin 5-HT2A or 5-HT2C receptors.[160][161][169] In addition, the serotonin 5-HT1B receptor has been found to be required for psilocybin's persisting antidepressant- and anxiolytic-like effects as well as acute hypolocomotion in animals.[170] In humans, ketanserin blocked psilocybin's hallucinogenic effects but not all of its cognitive and behavioral effects.[111] Serotonin 5-HT2C receptor activation and downstream inhibition of the mesolimbic dopamine pathway may be involved in the limited addictive potential of serotonergic psychedelics like psilocybin.[171]
In addition to its psychedelic effects, psilocin has been found to produce psychoplastogenic effects in animals, including dendritogenesis, spinogenesis, and synaptogenesis.[172][21][173] It has been found to promote neuroplasticity in the brain in a rapid, robust, and sustained manner with a single dose.[172][21] These effects appear to be mediated by intracellular serotonin 5-HT2A receptor activation.[172][21][174][173] The psychoplastogenic effects of psilocybin and other serotonergic psychedelics may be involved in their potential therapeutic benefits in the treatment of psychiatric disorders such as depression.[175][176][177] They may also be involved in the effects of microdosing.[178] Psilocin was also reported to act as a highly potent positive allosteric modulator of the tropomyosin receptor kinase B (TrkB), one of the receptors of brain-derived neurotrophic factor (BDNF),[172][16][179] but subsequent studies failed to reproduce these findings and instead found no interaction of psilocin with TrkB.[180] Relatedly, psilocybin has been found not to enhance but rather to inhibit hippocampal neurogenesis in rodents.[172]
Psilocybin produces profound anti-inflammatory effects mediated by serotonin 5-HT2A receptor activation in preclinical studies.[181][182][183] These effects have a potency similar to that of (R)-DOI, and its anti-inflammatory effects occur at far lower doses than those that produce hallucinogen-like effects in animals.[184][181][182][185] Psilocybin's anti-inflammatory effects might be involved in its potential antidepressant benefits and might also have other therapeutic applications, such as treatment of asthma and neuroinflammation.[181][182][186] They may also be involved in microdosing effects.[187][183] But psychedelics have been found to have anti-inflammatory effects only in the setting of preexisting inflammation and may be pro-inflammatory outside that context.[188] Psilocybin has been found to have a large, long-lasting impact on the intestinal microbiome and to influence the gut–brain axis in animals.[189][190][191][167][192][193] These effects are partially but not fully dependent on its activation of the serotonin 5-HT2A and/or 5-HT2C receptors.[167] Some of psilocybin's behavioral and potential therapeutic effects may be mediated by changes to the gut microbiome.[167][191][193] Transplantation of intestinal contents of psilocybin-treated rodents to untreated rodents resulted in behavioral changes consistent with those of psilocybin administration.[167]
Psilocybin and other psychedelics produce sympathomimetic effects, such as increased heart rate and blood pressure, by activating the serotonin 5-HT2A receptor.[194][195][196] Long-term repeated use of psilocybin may result in risk of cardiac valvulopathy and other complications by activating serotonin 5-HT2B receptors.[2][115][116][194][195]
There is little or no acute tolerance with psilocybin, and hence its duration is dictated by pharmacokinetics rather than by pharmacodynamics.[8][81] Conversely, tolerance and tachyphylaxis rapidly develop to psilocybin's psychedelic effects with repeated administration in humans.[2][197][160][111] In addition, there is cross-tolerance with the hallucinogenic effects of other psychedelics such as LSD.[2][197][160][111] Psilocybin produces downregulation of the serotonin 5-HT2A receptor in the brain in animals, an effect thought to be responsible for the development of tolerance to its psychedelic effects.[2][197][160][111] Serotonin 5-HT2A receptors appear to slowly return over the course of days to weeks after psilocybin administration.[2]
Pharmacokinetics
Absorption
There has been little research on psilocybin's bioavailability.[198] Its oral bioavailability, as its active form psilocin, was about 55.0% (± ~20%) relative to intravenous administration in one small older study (n=3).[198][21][6][82] After oral administration, psilocybin is detectable in the blood circulation within 20 to 40 minutes, and psilocin is detectable after 30 minutes.[6][27] The mean time to peak levels for psilocin is 1.05 to 3.71 hours in different studies, with most around 2 hours and the upper limit of 3.71 hours being an outlier.[198][199][27]
Psilocybin, in terms of psilocin, shows clear linear or dose-dependent pharmacokinetics.[198][8][21][6][81][200] Maximal concentrations of psilocin were 11 ng/mL, 17 ng/mL, and 21 ng/mL with oral psilocybin doses of 15, 25, and 30 mg psilocybin, respectively.[81] The maximal levels of psilocin have been found to range from 8.2 ng/mL to 37.6 ng/mL across a dose range of 14 to 42 mg.[199] The dose-normalized peak concentration of psilocin is about 0.8 ng/mL/mg.[198] The interindividual variability in the pharmacokinetics of psilocybin is relatively small.[81] There is a very strong positive correlation between dose and psilocin peak levels (R2 = 0.95).[199] The effects of food on the pharmacokinetics of psilocybin have not been reported and are unknown, but no clear sign of food effects has been observed in preliminary analyses.[198] It has also been said that food might delay absorption, reduce peak levels, and reduce bioavailability.[16]
Distribution
Psilocin, the active form of psilocybin, is extensively distributed to all tissues through the bloodstream.[6] Its volume of distribution is 505 to 1,267 L.[198] Psilocybin itself is hydrophilic due to its phosphate group and cannot easily cross the blood–brain barrier.[16][6] Conversely, psilocin is lipophilic and readily crosses the blood–brain barrier to exert effects in the central nervous system.[6] The plasma protein binding of psilocybin is 66% and hence it is moderately plasma protein-bound.[201]

Psilocin (4-HO-DMT) is a close positional isomer of bufotenin (5-HO-DMT), which shows peripheral selectivity, and might be expected to have similarly restricted lipophilicity and blood–brain barrier permeability.[202][203] But psilocin appears to form a tricyclic pseudo-ring system wherein its hydroxyl group and amine interact through hydrogen bonding.[202][203][204][205] This in turn makes psilocin much less polar, more lipophilic, and more able to cross the blood–brain barrier and exert central actions than it would be otherwise.[202][203][204][205] It may also protect psilocin from metabolism by monoamine oxidase (MAO).[202] In contrast, bufotenin is not able to achieve this pseudo-ring system.[202][203][204][205] Accordingly, bufotenin is less lipophilic than psilocin in terms of partition coefficient.[202][203] But bufotenin does still show significant central permeability and, like psilocybin, can produce robust hallucinogenic effects in humans.[203][204][206][207]
Metabolism

Psilocybin is dephosphorylated into its active form psilocin in the body and hence is a prodrug.[6][21][17] Psilocybin is metabolized in the intestines, liver, kidneys, blood, and other tissues and bodily fluids.[198][208][143] There is significant first-pass metabolism of psilocybin and psilocin with oral administration.[198][143] No psilocybin has been detected in the blood in humans after oral administration, suggesting virtually complete dephosphorylation into psilocin with the first pass.[198][21][17][143] It is also said to be converted 90% to 97% into psilocin.[209] The competitive phosphatase inhibitor β-glycerolphosphate, which inhibits psilocybin dephosphorylation, greatly attenuates the behavioral effects of psilocybin in rodents.[27][143][210] Psilocybin undergoes dephosphorylation into psilocin via the acidic environment of the stomach or the actions of alkaline phosphatase (ALP) and non-specific esterases in tissues and fluids.[26][208][27]
Psilocin is demethylated and oxidatively deaminated by monoamine oxidase (MAO), specifically monoamine oxidase A (MAO-A), into 4-hydroxyindole-3-acetaldehyde (4-HIAL or 4-HIA).[21][17][211] 4-HIAL is then further oxidated into 4-hydroxyindole-3-acetic acid (4-HIAA) by aldehyde dehydrogenase (ALDH) or into 4-hydroxytryptophol (4-HTOL or 4-HTP) by alcohol dehydrogenase (ALD).[21][17] Deamination of psilocin by MAO-A appears to be responsible for about 4% or 33% of its metabolism in different studies.[143][81][27] In contrast to psilocin, its metabolites 4-HIAA and 4-HTP showed no affinity for or activation of multiple serotonin receptors and are considered inactive.[21][16][143] Based on in vitro studies, it has been estimated that MAO-A is responsible for about 81% of psilocin's phase I hepatic metabolism.[211] Psilocin and its metabolites are also glucuronidated by UDP-glucuronyltransferases (UGTs).[198][21][17][143] UGT1A10 and UGT1A9 appear to be the most involved.[198][21][27] Psilocybin's glucuronidated metabolites include psilocin-O-glucuronide and 4-HIAA-O-glucuronide.[21][17][143] Approximately 80% of psilocin in blood plasma is in conjugated form, and conjugated psilocin levels are about fourfold higher than levels of free psilocin.[143][27] Plasma 4-HIAA levels are also much higher than those of free psilocin.[21]
Norpsilocin (4-HO-NMT), formed from psilocin via demethylation mediated by the cytochrome P450 enzyme CYP2D6, is known to occur in mice in vivo and with human recombinant CYP2D6 in vitro but was not detected in humans in vivo.[143] An oxidized psilocin metabolite of unknown chemical structure is also formed by hydroxyindole oxidase activity of CYP2D6.[143][27] Oxidized psilocin is possibly a quinone-type structure like psilocin iminoquinone (4-hydroxy-5-oxo-N,N-DMT) or psilocin hydroquinone (4,5-dihydroxy-N,N-DMT).[143][27] Additional metabolites formed by CYP2D6 may also be present.[143] Besides CYP2D6, CYP3A4 showed minor activity in metabolizing psilocin, though the produced metabolite is unknown.[143] Other cytochrome P450 enzymes besides CYP2D6 and CYP3A4 appear unlikely to be involved in psilocin metabolism.[143] CYP2D6 metabolizer phenotypes do not modify psilocin exposure in humans, suggesting that CYP2D6 is not critically involved in psilocin metabolism and is unlikely to result in interindividual differences in psilocin kinetics or effects.[16][143] Psilocybin and psilocin might inhibit CYP3A4 and CYP2A6 to some extent, respectively.[199]
Elimination
Psilocybin is eliminated 80% to 85% in urine and 15 to 20% in bile.[16] It is excreted mainly in urine as psilocin-O-glucuronide.[16][208] The drug was eliminated approximately 20% and 80% as psilocin O-glucuronide in different studies.[198][208][27][81] The amount excreted as unchanged psilocin in urine is 1.5 to 3.4%.[198][208][21][81] Studies conflict on the deaminated metabolites of psilocin, with one study finding that only 4% of psilocin is metabolized into 4-HIAA, 4-HIAL, and 4-HTOL[27] and another that psilocybin is excreted 33% in urine as 4-HIAA.[143][81] Findings also conflict on whether psilocybin can be detected in urine, with either no psilocybin excreted or 3% to 10% excreted as unchanged psilocybin.[209][78][198][17][27] A majority of psilocybin and its metabolites is excreted within three hours with oral administration and elimination is almost complete within 24 hours.[27][17][81]
The elimination half-life of psilocybin, as psilocin, is 2.1 to 4.7 hours on average (range 1.2–18.6 hours) orally and 1.2 hours (range 1.8–4.5 hours) intravenously.[198][199][21][27] Psilocin's elimination half-life in mice is 0.9 hours, much faster than in humans.[143] Psilocin O-glucuronide's half-life is about 4 hours in humans and approximately 1 hour in mice.[143]
No dose adjustment of psilocin is thought to be required as psilocin is inactivated mainly via metabolism as opposed to renal elimination.[198][16][81] Accordingly, glomerular filtration rate (GFR) did not affect the pharmacokinetics of psilocybin.[198][16][81]
Miscellaneous
The pharmacokinetics of administered psilocybin and psilocin in rodents, for instance in terms of psilocin tissue distribution kinetics, are described as very similar or identical, suggesting very rapid or near-immediate cleavage of psilocybin into psilocin.[212]
Psilocybin's psychoactive effects and duration are strongly correlated with psilocin levels.[8][81][21]
Single doses of psilocybin of 3 to 30 mg have been found to dose-dependently occupy the serotonin 5-HT2A receptor in humans as assessed by imaging studies.[21] The EC50 for occupancy of the serotonin 5-HT2A receptor by psilocin in terms of circulating levels has been found to be 1.97 ng/mL.[21]
Body weight and body mass index do not appear to affect psilocybin's pharmacokinetics.[8][16][81] This suggests that body weight-adjusted dosing of psilocybin is unnecessary and may actually be counterproductive, and that fixed-dosing should be preferred.[16][81] Similarly, age does not affect psilocybin's pharmacokinetics.[198] The influence of sex on psilocybin's pharmacokinetics has not been tested.[198]
Chemistry
Physical properties
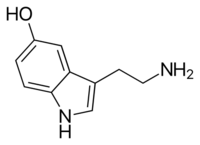
Psilocybin is a naturally occurring substituted tryptamine that features an indole ring linked to an aminoethyl substituent. It is structurally related to serotonin, a monoamine neurotransmitter that is a derivative of the amino acid tryptophan. Psilocybin is a member of the general class of tryptophan-based compounds that originally functioned as antioxidants in earlier life forms before assuming more complex functions in multicellular organisms, including humans.[213] Other related indole-containing psychedelic compounds include dimethyltryptamine, found in many plant species and in trace amounts in some mammals, and bufotenin, found in the skin of certain amphibians, especially the Colorado River toad.[214]: 10–13
Psilocybin is a white, crystalline solid that is soluble in water, methanol and ethanol but insoluble in nonpolar organic solvents such as chloroform and petroleum ether.[214]: 15 It has a melting point between 220 and 228 °C (428 and 442 °F),[122] and an ammonia-like taste.[215] Its pKa values are estimated to be 1.3 and 6.5 for the two successive phosphate hydroxy groups and 10.4 for the dimethylamine nitrogen, so it typically exists as a zwitterionic structure.[215] There are two known crystalline polymorphs of psilocybin, as well as reported hydrated phases.[216] Psilocybin rapidly oxidizes upon exposure to light—an important consideration when using it as an analytical standard.[217]
Structural analogues
Structural analogues of psilocybin (4-PO-DMT; O-phosphorylpsilocin) and psilocin (4-HO-DMT) include dimethyltryptamine (DMT), 5-hydroxytryptamine (5-HT), bufotenin (5-HO-DMT), 6-hydroxy-DMT, 4-AcO-DMT (psilacetin; O-acetylpsilocin), 4-PrO-DMT (O-propionylpsilocin), psilomethoxin (4-HO-5-MeO-DMT; 5-methoxypsilocin), ethocybin (4-PO-DET), baeocystin (4-PO-NMT), aeruginascin (4-PO-TMT), and norbaeocystin (4-PO-T), among others.
Laboratory synthesis
Albert Hofmann et al. were the first to synthesize psilocybin, in 1958. Since then, various chemists have improved the methods for laboratory synthesis and purification of psilocybin. In particular, Shirota et al. reported a novel method in 2003 for the synthesis of psilocybin at the gram scale from 4-hydroxyindole that does not require chromatographic purification. Fricke et al. described an enzymatic pathway for the synthesis of psilocybin and psilocin, publishing their results in 2017. Sherwood et al. significantly improved upon Shirota's method (producing at the kilogram scale while employing less expensive reagents), publishing their results in 2020.[218]
Analytical methods
Several relatively simple chemical tests—commercially available as reagent testing kits—can be used to assess the presence of psilocybin in extracts prepared from mushrooms. The drug produces a yellow color in the Marquis test and a green color in the Mandelin reagent.[219] Neither of these tests is specific for psilocybin; for example, the Marquis test will react with many classes of controlled drugs, such as those containing primary amino groups and unsubstituted benzene rings, including amphetamine and methamphetamine.[220] Ehrlich's reagent and DMACA reagent are used as chemical sprays to detect the drug after thin layer chromatography.[221] Many modern techniques of analytical chemistry have been used to quantify psilocybin levels in mushroom samples. Although the earliest methods commonly used gas chromatography, the high temperature required to vaporize the psilocybin sample before analysis causes it to spontaneously lose its phosphoryl group and become psilocin, making it difficult to chemically discriminate between the two drugs. In forensic toxicology, techniques involving gas chromatography coupled to mass spectrometry (GC–MS) are the most widely used due to their high sensitivity and ability to separate compounds in complex biological mixtures.[222] These techniques include ion mobility spectrometry,[223] capillary zone electrophoresis,[224] ultraviolet spectroscopy,[225] and infrared spectroscopy.[226] High-performance liquid chromatography (HPLC) is used with ultraviolet,[217] fluorescence,[227] electrochemical,[228] and electrospray mass spectrometric detection methods.[229]
Various chromatographic methods have been developed to detect psilocin in body fluids: the rapid emergency drug identification system (REMEDi HS), a drug screening method based on HPLC;[230] HPLC with electrochemical detection;[228][231] GC–MS;[232][230] and liquid chromatography coupled to mass spectrometry.[233] Although the determination of psilocin levels in urine can be performed without sample cleanup (i.e., removing potential contaminants that make it difficult to accurately assess concentration), the analysis in plasma or serum requires preliminary extraction followed by derivatization of the extracts in the case of GC–MS. A specific immunoassay has also been developed to detect psilocin in whole blood samples.[234] A 2009 publication reported using HPLC to quickly separate forensically important illicit drugs including psilocybin and psilocin, which were identifiable within about 30 seconds of analysis time.[235] But these analytical techniques to determine psilocybin concentrations in body fluids are not routinely available and not typically used in clinical settings.[48]
Natural occurrence
| Species | % psilocybin |
|---|---|
| P. azurescens | 1.78 |
| P. serbica | 1.34 |
| P. semilanceata | 0.98 |
| P. baeocystis | 0.85 |
| P. cyanescens | 0.85 |
| P. tampanensis | 0.68 |
| P. cubensis | 0.63 |
| P. weilii | 0.61 |
| P. hoogshagenii | 0.60 |
| P. stuntzii | 0.36 |
| P. cyanofibrillosa | 0.21 |
| P. liniformans | 0.16 |
Psilocybin is present in varying concentrations in over 200 species of Basidiomycota mushrooms. In a 2000 review on the worldwide distribution of hallucinogenic mushrooms, Gastón Guzmán and colleagues considered these to be distributed amongst the following genera: Psilocybe (116 species), Gymnopilus (14), Panaeolus (13), Copelandia (12), Hypholoma (6), Pluteus (6), Inocybe (6), Conocybe (4), Panaeolina (4), Gerronema (2), and Galerina (1 species).[237] Guzmán increased his estimate of the number of psilocybin-containing Psilocybe to 144 species in a 2005 review. The majority of these are found in Mexico (53 species), with the remainder distributed in the United States and Canada (22), Europe (16), Asia (15), Africa (4), and Australia and associated islands (19).[238] The diversity of psilocybin mushrooms is reported to have been increased by horizontal transfer of the psilocybin gene cluster between unrelated mushroom species.[239][240] In general, psilocybin-containing species are dark-spored, gilled mushrooms that grow in meadows and woods of the subtropics and tropics, usually in soils rich in humus and plant debris.[214]: 5 Psilocybin mushrooms occur on all continents, but the majority of species are found in subtropical humid forests.[237] Psilocybe species commonly found in the tropics include P. cubensis and P. subcubensis. P. semilanceata—considered by Guzmán to be the world's most widely distributed psilocybin mushroom[241]—is found in Europe, North America, Asia, South America, Australia and New Zealand, but is entirely absent from Mexico.[238] Although the presence or absence of psilocybin is not of much use as a chemotaxonomical marker at the familial level or higher, it is used to classify taxa of lower taxonomic groups.[242]

Both the caps and the stems contain psychoactive compounds, although the caps consistently contain more. The spores of these mushrooms do not contain psilocybin or psilocin.[223][244][245] The total potency varies greatly between species and even between specimens of a species collected or grown from the same strain.[246] Because most psilocybin biosynthesis occurs early in the formation of fruit bodies or sclerotia, younger, smaller mushrooms tend to have a higher concentration of the drug than larger, mature mushrooms.[247] In general, the psilocybin content of mushrooms is quite variable (ranging from almost nothing to 2.5% of the dry weight)[248][49]: 248 and depends on species, strain, growth and drying conditions, and mushroom size.[236]: 36–41, 52 Cultivated mushrooms have less variability in psilocybin content than wild mushrooms.[249] The drug is more stable in dried than fresh mushrooms; dried mushrooms retain their potency for months or even years,[236]: 51–5 while mushrooms stored fresh for four weeks contain only traces of the original psilocybin.[34]
The psilocybin contents of dried herbarium specimens of Psilocybe semilanceata in one study were shown to decrease with the increasing age of the sample: collections dated 11, 33, or 118 years old contained 0.84%, 0.67%, and 0.014% (all dry weight), respectively.[250] Mature mycelia contain some psilocybin, while young mycelia (recently germinated from spores) lack appreciable amounts.[251] Many species of mushrooms containing psilocybin also contain lesser amounts of the analog compounds baeocystin and norbaeocystin,[236]: 38 chemicals thought to be biogenic precursors.[40]: 170 Although most species of psilocybin-containing mushrooms bruise blue when handled or damaged due to the oxidization of phenolic compounds, this reaction is not a definitive method of identification or determining a mushroom's potency.[246][236]: 56–58
Biosynthesis
Isotopic labeling experiments from the 1960s suggested that the biosynthesis of psilocybin was a four-step process:[252]
- Decarboxylation of tryptophan to tryptamine
- N,N-Dimethylation of tryptamine at the N9 position to dimethyltryptamine
- 4-Hydroxylation of dimethyltryptamine to psilocin
- O-Phosphorylation of psilocin to psilocybin
This process can be seen in the following diagram:[253]
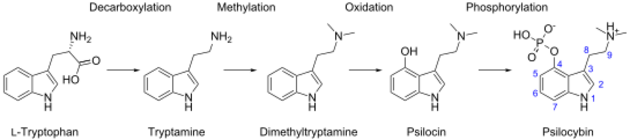
More recent research has demonstrated that—at least in P. cubensis—O-phosphorylation is in fact the third step, and that neither dimethyltryptamine nor psilocin are intermediates.[253] The sequence of the intermediate steps has been shown to involve four enzymes (PsiD, PsiH, PsiK, and PsiM) in P. cubensis and P. cyanescens, although it is possible that the biosynthetic pathway differs between species.[214]: 12–13 [253] These enzymes are encoded in gene clusters in Psilocybe, Panaeolus, and Gymnopilus.[240]
Escherichia coli has been genetically modified to manufacture large amounts of psilocybin.[254] Psilocybin can be produced de novo in GM yeast.[255][256]
History
Early

The use of psilocybin mushrooms in religious ceremonies dating back thousands of years is contested.[9] Despite popular narratives portraying psychedelics as ancient, widespread, and primarily used by shamans for therapeutic healing, anthropological and historical research shows their traditional use was often limited, recent, and culturally specific, with modern Western interpretations largely shaped by idealization, tourism, and ideological agendas.[9] Reliable evidence shows that psilocybin mushrooms were used ritualistically in pre-Columbian Mexico but were otherwise rare, with most claims of ancient widespread use exaggerated or misinterpreted.[9]
It has been argued that the Tassili Mushroom Figure, discovered in Tassili, Algeria, is evidence of an early psilocybin-containing mushroom cult.[257] 6,000-year-old pictographs discovered near Villar del Humo, Spain, illustrate several mushrooms that have been argued to be Psilocybe hispanica, a hallucinogenic species native to the area.[258] Some scholars have also interpreted archaeological artifacts from Mexico and the so-called Mayan "mushroom stones" of Guatemala as evidence of ritual and ceremonial use of psychoactive mushrooms in the Mayan and Aztec cultures.[236]: 11
After Spanish conquistadors of the New World arrived in the 16th century, chroniclers reported mushroom use by the natives for ceremonial and religious purposes. According to the Dominican friar Diego Durán in The History of the Indies of New Spain (published c. 1581), mushrooms were eaten in festivities conducted on the occasion of Aztec emperor Moctezuma II's accession to the throne in 1502. The Franciscan friar Bernardino de Sahagún wrote of witnessing mushroom use in the Florentine Codex (published 1545–1590),[259]: 164 saying that some merchants celebrated upon returning from a successful business trip by consuming mushrooms to evoke revelatory visions.[260]: 118 After the defeat of the Aztecs, the Spanish forbade traditional religious practices and rituals they considered "pagan idolatry", including ceremonial mushroom use. For the next four centuries, the Indians of Mesoamerica hid their use of entheogens from the Spanish authorities.[259]: 165
Dozens of species of psychedelic mushrooms are found in Europe, but there is little documented usage of them in Old World history besides the use of Amanita muscaria among Siberian peoples.[261][262] The few existing accounts that mention psilocybin mushrooms typically lack sufficient information to allow species identification, focusing on their effects. For example, Flemish botanist Carolus Clusius (1526–1609) described the bolond gomba ("crazy mushroom"), used in rural Hungary to prepare love potions. English botanist John Parkinson included details about a "foolish mushroom" in his 1640 herbal Theatricum Botanicum.[263]: 10–12 The first reliably documented report of intoxication with Psilocybe semilanceata—Europe's most common and widespread psychedelic mushroom—involved a British family in 1799, who prepared a meal with mushrooms they had picked in London's Green Park.[263]: 16
Modern


American banker and amateur ethnomycologist R. Gordon Wasson and his wife, Valentina P. Wasson, a physician, studied the ritual use of psychoactive mushrooms by the native population in the Mazatec village Huautla de Jiménez, Mexico. In 1957, Wasson described the psychedelic visions he experienced during these rituals in "Seeking the Magic Mushroom", an article published in the American weekly Life magazine.[264] Later the same year they were accompanied on a follow-up expedition by French mycologist Roger Heim, who identified several of the mushrooms as Psilocybe species.[265]
Heim cultivated the mushrooms in France and sent samples for analysis to Albert Hofmann, a chemist employed by the Swiss pharmaceutical company Sandoz. Hofmann—who had synthesized lysergic acid diethylamide (LSD) in 1938—led a research group that isolated and identified the psychoactive alkaloids psilocybin and psilocin from Psilocybe mexicana, publishing their results in 1958.[260]: 128 The team was aided in the discovery process by Hofmann's willingness to ingest mushroom extracts to help verify the presence of the active compounds.[260]: 126–127
Next, Hofmann's team synthesized several structural analogs of these compounds to examine how these structural changes affect psychoactivity. This research led to the development of ethocybin and CZ-74. Because these compounds' physiological effects last only about three and a half hours (about half as long as psilocybin's), they proved more manageable for use in psycholytic therapy.[49]: 237 Sandoz also marketed and sold pure psilocybin under the name Indocybin to clinicians and researchers worldwide.[259]: 166 There were no reports of serious complications when psilocybin was used in this way.[78]
In the early 1960s, Harvard University became a testing ground for psilocybin through the efforts of Timothy Leary and his associates Ralph Metzner and Richard Alpert (who later changed his name to Ram Dass). Leary obtained synthesized psilocybin from Hofmann through Sandoz Pharmaceuticals. Some studies, such as the Concord Prison Experiment, suggested promising results using psilocybin in clinical psychiatry.[50][266] But according to a 2008 review of safety guidelines in human hallucinogenic research, Leary's and Alpert's well-publicized termination from Harvard and later advocacy of hallucinogen use "further undermined an objective scientific approach to studying these compounds".[267] In response to concerns about the increase in unauthorized use of psychedelic drugs by the general public, psilocybin and other hallucinogenic drugs were unfavorably covered in the press and faced increasingly restrictive laws. In the U.S., laws passed in 1966 that prohibited the production, trade, or ingestion of hallucinogenic drugs; Sandoz stopped producing LSD and psilocybin the same year.[268] In 1970, Congress passed "The Federal Comprehensive Drug Abuse Prevention and Control Act" that made LSD, peyote, psilocybin, and other hallucinogens illegal to use for any purpose, including scientific research.[269] United States politicians' agenda against LSD usage had swept psilocybin along with it into the Schedule I category of illicit drugs. Such restrictions on the use of these drugs in human research made funding for such projects difficult to obtain, and scientists who worked with psychedelic drugs faced being "professionally marginalized".[270] Although Hofmann tested these compounds on himself, he never advocated their legalization or medical use. In his 1979 book LSD—mein Sorgenkind (LSD—My Problem Child), he described the problematic use of these hallucinogens as inebriants.[260]: 79–116
Despite the legal restrictions on psilocybin use, the 1970s witnessed the emergence of psilocybin as the "entheogen of choice".[271]: 276 This was due in large part to wide dissemination of information on the topic, which included works such as those by Carlos Castaneda and several books that taught the technique of growing psilocybin mushrooms. One of the most popular of the latter group, Psilocybin: Magic Mushroom Grower's Guide, was published in 1976 under the pseudonyms O. T. Oss and O. N. Oeric by Jeremy Bigwood, Dennis J. McKenna, K. Harrison McKenna, and Terence McKenna. Over 100,000 copies were sold by 1981.[272] As ethnobiologist Jonathan Ott explains, "These authors adapted San Antonio's technique (for producing edible mushrooms by casing mycelial cultures on a rye grain substrate; San Antonio 1971) to the production of Psilocybe [Stropharia] cubensis. The new technique involved the use of ordinary kitchen implements, and for the first time the layperson was able to produce a potent entheogen in his own home, without access to sophisticated technology, equipment or chemical supplies."[271]: 290 San Antonio's technique describes a method to grow the common edible mushroom Agaricus bisporus.[273]
Because of lack of clarity about laws concerning psilocybin mushrooms, specifically in the form of sclerotia (also known as "truffles"), in the late 1990s and early 2000s European retailers commercialized and marketed them in smartshops in the Netherlands, the UK, and online. Several websites[lower-alpha 5] emerged that contributed to the accessibility of information on the mushrooms' description, use, and effects, and users exchanged mushroom experiences. Since 2001, six EU countries have tightened their legislation on psilocybin mushrooms in response to concerns about their prevalence and increasing usage.[33] In the 1990s, hallucinogens and their effects on human consciousness were again the subject of scientific study, particularly in Europe. Advances in neuropharmacology and neuropsychology and the availability of brain imaging techniques have provided impetus for using drugs like psilocybin to probe the "neural underpinnings of psychotic symptom formation including ego disorders and hallucinations".[41] Recent studies in the U.S. have attracted attention from the popular press and brought psilocybin back into the limelight.[274][275]
Society and culture
Usage

A 2009 national survey of drug use by the US Department of Health and Human Services concluded that the number of first-time psilocybin mushroom users in the United States was roughly equivalent to the number of first-time users of cannabis.[276] A June 2024 report by the RAND Corporation suggests the total number of use days for psychedelics is two orders of magnitude smaller than it is for cannabis, and unlike people who use cannabis and many other drugs, infrequent users of psychedelics account for most of the total days of use.[277] The RAND Corporation report suggests psilocybin mushrooms may be the most prevalent psychedelic drug among U.S. adults.[277]
In European countries, the lifetime prevalence estimates of psychedelic mushroom usage among young adults (15–34 years) range from 0.3% to 14.1%.[278]
In modern Mexico, traditional ceremonial use survives among several indigenous groups, including the Nahuas, the Matlatzinca, the Totonacs, the Mazatecs, Mixes, Zapotecs, and the Chatino. Although hallucinogenic Psilocybe species are abundant in Mexico's low-lying areas, most ceremonial use takes places in mountainous areas of elevations greater than 1,500 meters (4,900 ft). Guzmán suggests this is a vestige of Spanish colonial influence from several hundred years earlier, when mushroom use was persecuted by the Catholic Church.[279]
Legal status
Advocacy for tolerance
Despite being illegal to possess without authorization in many Western countries, such as the UK, Australia, and some U.S. states, less conservative governments nurture the legal possession and supply of psilocybin and other psychedelic drugs. In Amsterdam, authorities provide education on and promote the safe use of psychedelic drugs, such as psilocybin, to reduce public harm.[280] Similarly, religious groups like America's Uniao do Vegetal (UDV)[281] use psychedelics in traditional ceremonies.[282] A report from the U.S. Government Accountability Office (GAO) notes that people may petition the DEA for exemptions to use psilocybin for religious purposes.[283]
From 1 July 2023, the Australian medicines regulator has permitted psychiatrists to prescribe psilocybin for the therapeutic treatment of treatment-resistant depression.[284]
Advocates of legalization argue there is a lack of evidence of harm,[285][286] and potential use in treating certain mental health conditions. Research is difficult to conduct because of the legal status of psychoactive substances.[287] Advocates of legalization also promote the utility of "ego dissolution"[281] and argue bans are cultural discrimination against traditional users.[288]
In 2024, after calls for regulatory and legal change to expand terminally ill populations' access to controlled substances, two legal cases related to expanded access began moving through the federal courts under right-to-try law. The Advanced Integrative Medicine Science (AIMS) Institute in concert with the NPA filed a series of lawsuits seeking both the rescheduling of and expanded right-to-try access to psilocybin.[289]
Research
Psychiatric and other disorders
Psilocybin has been a subject of clinical research since the early 1960s, when the Harvard Psilocybin Project evaluated the potential value of psilocybin as a treatment for certain personality disorders.[290] Beginning in the 2000s, psilocybin has been investigated for its possible role in the treatment of nicotine dependence, alcohol dependence, obsessive–compulsive disorder (OCD), cluster headache, cancer-related existential distress,[218][291] anxiety disorders, and certain mood disorders.[259]: 179–81 [292][293] It is also being studied in people with Parkinson's disease.[294][295] In 2018, the United States Food and Drug Administration (FDA) granted breakthrough therapy designation for psilocybin-assisted therapy for treatment-resistant depression.[296][297] A systematic review published in 2021 found that the use of psilocybin as a pharmaceutical substance was associated with reduced intensity of depression symptoms.[298] The role of psilocybin as a possible psychoplastogen is also being examined.[175][176][177] It is under development by Compass Pathways, Cybin, and several other companies.[299][300]
Depression
Clinical trials, including both open-label trials and double-blind randomized controlled trials (RCTs), have found that single doses of psilocybin produce rapid and long-lasting antidepressant effects outperforming placebo in people with major depressive disorder and treatment-resistant depression.[301] Combined with brief psychological support in a Phase II trial, it has been found to produce dose-dependent improvements in depressive symptoms, with 25 mg (a moderate dose) more effective than 10 mg (a low dose), and 10 mg more effective than 1 mg (non-psychoactive and equivalent to placebo).[301][302] The antidepressant effects of psilocybin with psychological support have been found to last at least 6 weeks following a single dose.[301][302][303][304]
However, some trials have not found psilocybin to significantly outperform placebo in the treatment of depression.[301] In addition, a Phase II trial found that two 25 mg doses of psilocybin 3 weeks apart versus daily treatment with the selective serotonin reuptake inhibitor (SSRI) escitalopram (Lexapro) for 6 weeks (plus two putatively non-psychoactive 1 mg doses of psilocybin 3 weeks apart) did not show a statistically significant difference in reduction of depressive symptoms between groups.[301][305] However, reductions in depressive symptoms were numerically greater with psilocybin, some secondary measures favored psilocybin, and the rate of remission was statistically higher with psilocybin (57% with psilocybin vs. 28% with escitalopram).[301][305] In any case, the antidepressant effect size of psilocybin over escitalopram appears to be small.[306]
Functional unblinding by their psychoactive effects and positive psychological expectancy effects (i.e., the placebo effect) are major limitations and sources of bias of clinical trials of psilocybin and other psychedelics for treatment of depression.[307][308][309][310] Relatedly, most of the therapeutic benefit of conventional antidepressants like the SSRIs for depression appears to be attributable to the placebo response.[311][312] It has been proposed that psychedelics like psilocybin may in fact act as active "super placebos" when used for therapeutic purposes.[313][314] Psilocybin has not received regulatory approval for medical use in the United States.[15][301][87]
In a 2024 meta-analysis of RCTs of psychedelics and escitalopram for treatment of depression, only "high-dose" psilocybin (≥20 mg) significantly outperformed escitalopram in improving depressive symptoms.[306] It showed a large effect size over placebo, but a small effect size over escitalopram.[306] A 2025 meta-analysis found a moderate effect size advantage of psilocybin relative to placebo.[315] A 2024 network meta-analysis of RCTs of therapies for treatment-resistant depression, with effectiveness measures being response and remission rates, likewise found that psilocybin was more effective than placebo and, considering both effectiveness and tolerability or safety, recommended it as a first-line therapy, along with ketamine, esketamine, and electroconvulsive therapy (ECT).[316] However, the quality of evidence was generally rated as low or very low.[316] Meta-analyses of psychedelics for depression and other psychiatric conditions have found that psilocybin has the greatest number of studies and the most evidence of benefit, relative to other psychedelics like ayahuasca and LSD.[306][317][87][318]
Preliminary meta-analyses suggest that improvements in depressive symptoms with psilocybin are dose-dependent and that higher doses may result in greater improvements than lower doses.[319][320][321] One meta-analysis found that the highest assessed dose in clinical trials, 30 to 35 mg per 70 kg body weight, was the most effective, with an effect size (Hedges' g) of 3.1 (relative to 1.3 overall), but based on only one study for that dosing subgroup.[319] This meta-analysis included both RCTs and prospective open-label studies, and calculated effect sizes by comparing to the placebo group or by using pre-treatment (baseline) values.[319] Another meta-analysis, which included only RCTs, found that 25 mg was the most effective dose, relative to lower doses like 10 mg and 0.215 mg/kg body weight (~15 mg for a 70-kg person).[321] A third meta-analysis found that half of psilocybin's maximal antidepressant effect occurred with a dose of about 10 mg per 70 kg body weight, while 95% of the maximal effect occurred at a dose of about 41 mg per 70 kg body weight, and that higher doses might especially be better for treatment-resistant depression.[320] The risk of adverse effects was also greater with higher doses.[320]
A 2025 network meta-analysis of RCTs of psilocybin for depression found that it did not significantly improve depression scores relative to placebo on day 2 post-dose but did improve them day 8 and day 15 post-dose.[321] Depressive symptoms were improved only slightly more with psilocybin than with placebo.[321] Another 2024 meta-analysis found that depressive symptoms were improved on days 2, 14, and 42, with similar effect sizes.[322] In the previously described dose-ranging Phase II trial of psilocybin for depression, the time to median depressive event after administration of psilocybin was 92 to 189 days for 25 mg, 43 to 83 days for 10 mg, and 21 to 62 days for 1 mg, depending on the analysis.[304] Repeated dosing of psilocybin is being explored for maximization and maintenance of depressive symptom improvement, with preliminary effectiveness observed.[14][323][324]
In June 2025, Compass Pathways, which is developing psilocybin for treatment-resistant depression, announced the results of a Phase III clinical trial of single-dose 25 mg psilocybin (COMP360) versus placebo.[325][326][327][328] Psilocybin met the primary endpoint of a significant reduction in depressive scores on the Montgomery-Asberg Depression Rating Scale (MADRS) relative to placebo.[325][326] At the 6-week point, there was a 3.6-point reduction in depressive symptoms on the scale compared to placebo.[326] The degree of improvement over placebo was small and below expectations: a minimum advantage of at least 5 points over placebo had been expected and deemed acceptable, for instance by the company's investors.[325][326][327][328] After Compass Pathways's announcement, the company's stock price fell by 36%.[325][326][327][328] Since October 2024, upon initially delaying the announcement of the results, Compass Pathways has laid off 30% of its staff and stopped all preclinical work unrelated to COMP360.[326] A second Phase III trial by Compass Pathways involving multiple different doses of psilocybin is also underway, with results expected next year.[326] Questions remain concerning the durability of psilocybin's antidepressant effects, the scalability of its treatment delivery, and regulatory uncertainity.[325]
Most clinical trials of psilocybin for depression have had financial conflicts of interest and significant risk of bias.[315] The British critical psychiatrist Joanna Moncrieff has critiqued the use and study of psychedelics like psilocybin for treatment of psychiatric disorders, highlighting concerns including excessive hype around these drugs, questionable biologically-based theories of benefit, blurred lines between medical and recreational use, flawed clinical trial findings, financial conflicts of interest, strong expectancy effects and large placebo responses, small and short-term benefits over placebo, and their potential for difficult and potentially destabilizing experiences and adverse effects, among others.[329]
See also
- List of entheogens
- Stoned ape theory
- Soma (drink)
Notes
- ↑ Synonyms and alternate spellings of psilocybin include 4-PO-DMT (PO: phosphate; DMT: dimethyltryptamine), psilocybine, psilocibin, psilocybinum, psilotsibin, and psilocin phosphate ester, among others.[1]
- ↑ The academic communities' approval for the methodology employed is exemplified by the quartet of commentaries published in the journal Psychopharmacology titled "Commentary on: Psilocybin can occasion mystical-type experiences having substantial and sustained personal meaning and spiritual experience by Griffiths et al.", by HD Kleber (pp. 291–292), DE Nichols (pp. 284–286), CR Schuster (pp. 289–290), and SH Snyder (pp. 287–288).
- ↑ Percentages are derived from a non-blind clinical study of 30 individuals who were given a dose of 8 to 12 mg of psilocybin; from Passie (2002),[78] citing Quentin (1960).[79]
- ↑ One of the reported fatalities, that of a 22-year-old French man who died in 1993,[125] was later challenged in the literature by Jochen Gartz and colleagues, who concluded "the few reported data concerning the victim are insufficient to exclude other possible causes of the fatality".[126]
- ↑ The EMCDDA lists the general-purpose websites Erowid, Lycaeum, Mycotopia, The Shroomery, MushroomJohn and The Entheogen Review. Regional sites focusing on hallucinogenic mushrooms listed were Copenhagen Mushroom Link (Denmark), Champis (France), Daath (Hungary), Delysid (Spain), Enteogeneos (Portugal), Kouzelné houbičky (Czech Republic), Norshroom (Norway), Planetahongo (Spain), Svampinfo (Sweden), and Taikasieniforum (Finland). It also listed Magic-Mushrooms.net. The report detailed several additional sites selling spore prints in 2006, but noted that many of these had ceased operation.
References
- ↑ "Psilocybine – Compound Summary". PubChem. National Library of Medicine. https://pubchem.ncbi.nlm.nih.gov/compound/10624.
- ↑ 2.00 2.01 2.02 2.03 2.04 2.05 2.06 2.07 2.08 2.09 2.10 2.11 2.12 2.13 "DARK Classics in Chemical Neuroscience: Psilocybin". ACS Chem Neurosci 9 (10): 2438–2447. October 2018. doi:10.1021/acschemneuro.8b00186. PMID 29956917. https://shaunlacob.com/wp-content/uploads/2020/12/DC-PSILO.pdf.
- ↑ "Psilocybin" (in en). PubChem. U.S. National Library of Medicine. https://pubchem.ncbi.nlm.nih.gov/compound/Psilocybin.
- ↑ 4.0 4.1 "Psilocybin desynchronizes the human brain". Nature 632 (8023): 131–138. August 2024. doi:10.1038/s41586-024-07624-5. PMID 39020167. Bibcode: 2024Natur.632..131S.
- ↑ 5.0 5.1 "Psilocybin: from ancient magic to modern medicine". J Antibiot (Tokyo) 73 (10): 679–686. October 2020. doi:10.1038/s41429-020-0311-8. PMID 32398764. https://www.researchgate.net/publication/341321407. "Total psilocybin and psilocin levels in species known to be used recreationally varied from 0.1% to nearly 2% by dry weight [8]. The medium oral dose of psilocybin is 4–8 mg, which elicits the same symptoms as the consumption of about 2 g of dried Psilocybe Mexicana [9].".
- ↑ 6.00 6.01 6.02 6.03 6.04 6.05 6.06 6.07 6.08 6.09 6.10 6.11 6.12 6.13 6.14 6.15 Cite error: Invalid
<ref>tag; no text was provided for refs namedMacCallumLoPistawka2022 - ↑ 7.0 7.1 "Acute Adverse Effects of Therapeutic Doses of Psilocybin: A Systematic Review and Meta-Analysis". JAMA Network Open 7 (4). April 2024. doi:10.1001/jamanetworkopen.2024.5960. PMID 38598236. "When selecting adverse event profile rates, the shortest time period available was selected and analyzed (eg, day 1 instead of day 30) since the half-life of psilocin is 3 ± 1.1 hours when taken orally and the duration of action can range between 3 to 12 hours.12,13".
- ↑ 8.00 8.01 8.02 8.03 8.04 8.05 8.06 8.07 8.08 8.09 8.10 8.11 8.12 8.13 8.14 8.15 8.16 8.17 8.18 Cite error: Invalid
<ref>tag; no text was provided for refs namedHolzeSinghLiechti2024 - ↑ 9.0 9.1 9.2 9.3 "The ancient psychedelics myth: 'People tell tourists the stories they think are interesting for them'" (in en-GB). The Guardian. 2025-05-01. ISSN 0261-3077. https://www.theguardian.com/science/2025/may/01/the-ancient-psychedelics-myth-people-tell-tourists-the-stories-they-think-are-interesting-for-them.
- ↑ 10.0 10.1 "Psilocybin for clinical indications: A scoping review". J Psychopharmacol 38 (10): 839–845. October 2024. doi:10.1177/02698811241269751. PMID 39135496.
- ↑ "The Australia story: Current status and future challenges for the clinical applications of psychedelics". British Journal of Pharmacology. December 2024. doi:10.1111/bph.17398. PMID 39701143.
- ↑ "From prohibited to prescribed: The rescheduling of MDMA and psilocybin in Australia". Drug Science, Policy and Law 9. 2023. doi:10.1177/20503245231198472. ISSN 2050-3245.
- ↑ "Uncovering Psychedelics: From Neural Circuits to Therapeutic Applications". Pharmaceuticals (Basel) 18 (1): 130. January 2025. doi:10.3390/ph18010130. PMID 39861191.
- ↑ 14.0 14.1 "The role of psilocybin in depressive disorders". Curr Med Res Opin 40 (10): 1793–1808. October 2024. doi:10.1080/03007995.2024.2396536. PMID 39177339.
- ↑ 15.0 15.1 15.2 "Psilocybin - COMPASS Pathways". 15 May 2024. https://adisinsight.springer.com/drugs/800050861.
- ↑ 16.00 16.01 16.02 16.03 16.04 16.05 16.06 16.07 16.08 16.09 16.10 16.11 16.12 16.13 16.14 16.15 16.16 16.17 16.18 "Psilocybin and hallucinogenic mushrooms". CNS Spectr 29 (6): 611–632. January 2025. doi:10.1017/S1092852924002487. PMID 39789676. "Upon their activation by psilocin, 5-HT2A receptors initiate complex cascades of downstream signaling. The activation of both canonical Gq/11 and β-arrestin-2 seems necessary to produce psychedelic effects,133 and so is the coactivation of Gi/o and Src tyrosine kinase.134 These specific pathways are thought to differentiate 5-HT2A receptor agonists with psychedelic properties from other agonists of the same receptor such as ergoline and lisuride that do not have hallucinogenic effects. [...] Although strong evidence supports that 5-HT2A activity mediates most of psilocin’s psychedelic properties, this substituted tryptamine also binds to many other receptors135–137. In fact, psilocin’s binding affinity is even higher for some other serotonin receptors such as 5-HT2C, 5-HT1A, and 5-HT2B137. It is currently difficult to determine the clinical significance of psilocin’s interaction with these receptors. Although they do not seem to contribute to the hallucinogenic properties of psilocin, these other serotonin receptors could potentially play a role in mediating its therapeutic effect.136, 138–140 [...] Psilocin has a very low affinity for the serotonin transporter (SERT), and it does not interact directly with the norepinephrine transporter (NET) or the dopamine transporter (DAT).137 Although it has the potential to bind with D1 and D3 receptors, it has no direct activity on the widespread D2 receptors.137 It does not interact with adrenergic, opioid, muscarinic, histamine, or cannabinoid receptors.137".
- ↑ 17.00 17.01 17.02 17.03 17.04 17.05 17.06 17.07 17.08 17.09 17.10 17.11 Cite error: Invalid
<ref>tag; no text was provided for refs namedLoweToyangSteele2021 - ↑ "The Australia story: Current status and future challenges for the clinical applications of psychedelics". British Journal of Pharmacology. December 2024. doi:10.1111/bph.17398. PMID 39701143.
- ↑ "From prohibited to prescribed: The rescheduling of MDMA and psilocybin in Australia". Drug Science, Policy and Law 9. 2023. doi:10.1177/20503245231198472. ISSN 2050-3245.
- ↑ "Australia Legalizes Psychedelics for Use in Depression, PTSD Therapy". Psychiatric News 58 (9). 1 September 2023. doi:10.1176/appi.pn.2023.09.9.20. ISSN 0033-2704. https://psychiatryonline.org/doi/10.1176/appi.pn.2023.09.9.20. Retrieved 17 May 2025.
- ↑ 21.00 21.01 21.02 21.03 21.04 21.05 21.06 21.07 21.08 21.09 21.10 21.11 21.12 21.13 21.14 21.15 21.16 21.17 21.18 21.19 21.20 21.21 21.22 21.23 21.24 21.25 21.26 21.27 21.28 "Psilocybin in neuropsychiatry: a review of its pharmacology, safety, and efficacy". CNS Spectr 28 (4): 416–426. August 2023. doi:10.1017/S1092852922000888. PMID 35811423. https://www.cambridge.org/core/services/aop-cambridge-core/content/view/AA1FB4F49C14BA3F398238D6E5A3947A/S1092852922000888a.pdf/div-class-title-psilocybin-in-neuropsychiatry-a-review-of-its-pharmacology-safety-and-efficacy-div.pdf.
- ↑ 22.0 22.1 22.2 22.3 "Dosing Psychedelics and MDMA". Disruptive Psychopharmacology. Curr Top Behav Neurosci. 56. 2022. pp. 3–21. doi:10.1007/7854_2021_270. ISBN 978-3-031-12183-8. https://www.researchgate.net/publication/355943062.
- ↑ 23.0 23.1 23.2 23.3 "Acute psychological and physiological effects of psilocybin in healthy humans: a double-blind, placebo-controlled dose-effect study". Psychopharmacology 172 (2): 145–156. March 2004. doi:10.1007/s00213-003-1640-6. PMID 14615876. http://www.maps.org/research-archive/w3pb/2004/2004_Hasler_20465_2.pdf. Retrieved April 10, 2019.
- ↑ "From psychiatry to neurology: Psychedelics as prospective therapeutics for neurodegenerative disorders". J Neurochem 162 (1): 89–108. July 2022. doi:10.1111/jnc.15509. PMID 34519052. "One dosing method of psychedelics is the use of so called “microdoses”—very low concentrations of various psychedelics that do not reach the threshold of perceivable behavioral effects. This is usually 10% of active recreational doses (e.g., 10–15 µg of LSD, or 0.1–0.3 g of dry “magic mushrooms”) taken up to three times per week.".
- ↑ 25.0 25.1 "Harm potential of magic mushroom use: a review". Regul Toxicol Pharmacol 59 (3): 423–429. April 2011. doi:10.1016/j.yrtph.2011.01.006. PMID 21256914. https://psilosybiini.info/paperit/Harm%20potential%20of%20magic%20mushroom%20use,%20A%20review%20(van%20Amsterdam%20et%20al.,%202011).pdf.
- ↑ 26.0 26.1 "A journey with psychedelic mushrooms: From historical relevance to biology, cultivation, medicinal uses, biotechnology, and beyond". Biotechnol Adv 69. December 2023. doi:10.1016/j.biotechadv.2023.108247. PMID 37659744.
- ↑ 27.00 27.01 27.02 27.03 27.04 27.05 27.06 27.07 27.08 27.09 27.10 27.11 27.12 27.13 27.14 27.15 27.16 27.17 27.18 27.19 27.20 27.21 27.22 27.23 "Psilocybin - summary of knowledge and new perspectives". Eur Neuropsychopharmacol 24 (3): 342–356. March 2014. doi:10.1016/j.euroneuro.2013.12.006. PMID 24444771. https://www.researchgate.net/publication/259517753.
- ↑ "Exploring Psilocybe cubensis Strains: Cultivation Techniques, Psychoactive Compounds, Genetics and Research Gaps". Journal of Fungi 11 (2): 99. January 2025. doi:10.3390/jof11020099. PMID 39997393.
- ↑ "Determination of psilocybin and psilocin content in multiple Psilocybe cubensis mushroom strains using liquid chromatography - tandem mass spectrometry". Anal Chim Acta 1288. February 2024. doi:10.1016/j.aca.2023.342161. PMID 38220293. Bibcode: 2024AcAC.128842161G. "A method for clinical potency determination of psilocybin and psilocin in hallucinogenic mushroom species Psilocybe cubensis was developed using liquid chromatography with tandem mass spectrometry (LC-MS/MS). Five strains of dried, intact mushrooms were obtained and analyzed: Blue Meanie, Creeper, B-Plus, Texas Yellow, and Thai Cubensis. [...] From most to least potent, the study found that the average total psilocybin and psilocin concentrations for the Creeper, Blue Meanie, B+, Texas Yellow, and Thai Cubensis strains were 1.36, 1.221, 1.134, 1.103, and 0.879 % (w/w), respectively.".
- ↑ "Extensive Collection of Psychotropic Mushrooms with Determination of Their Tryptamine Alkaloids". Int J Mol Sci 23 (22). November 2022. doi:10.3390/ijms232214068. PMID 36430546.
- ↑ Albert Hofmann (1968). "Psychotomimetic Agents". Drugs Affecting the Central Nervous System. 2. New York: M. Dekker. pp. 169–235. OCLC 245452885. https://archive.org/details/drugsaffectingce0000edit/page/169/mode/1up. "Psilocin is approximately 1.4 times as potent as psilocybin. This ratio is the same as that of the molecular weights of the two drugs."
- ↑ "Comparison of psilocin with psilocybin, mescaline and LSD-25". Psychopharmacologia 3 (3): 219–223. 1962. doi:10.1007/BF00412109. PMID 14007905. "Psilocin is approximately 1.4 times as potent as psilocybin. This ratio is the same as that of the molecular weights of the two drugs.".
- ↑ 33.0 33.1 Hallucinogenic Mushrooms: An Emerging Trend Case Study (Report). Lisbon, Portugal: European Monitoring Centre for Drugs and Drug Addiction (EMCDDA). 2006. ISBN 92-9168-249-7. http://www.emcdda.europa.eu/attachements.cfm/att_31215_EN_TP_Hallucinogenic_mushrooms.pdf. Retrieved September 8, 2011.
- ↑ 34.00 34.01 34.02 34.03 34.04 34.05 34.06 34.07 34.08 34.09 34.10 "Harm potential of magic mushroom use: a review". Regulatory Toxicology and Pharmacology 59 (3): 423–429. April 2011. doi:10.1016/j.yrtph.2011.01.006. PMID 21256914. http://dl.dropbox.com/u/85192141/2011-amsterdam.pdf.
- ↑ "A "Trip" to the Intensive Care Unit: An Intravenous Injection of Psilocybin". J Acad Consult Liaison Psychiatry 62 (3): 370–371. 2021. doi:10.1016/j.jaclp.2020.12.012. PMID 34102133.
- ↑ "Intravenous injection of mushrooms". Med J Aust 140 (3): 182. February 1984. doi:10.5694/j.1326-5377.1984.tb103978.x. PMID 6694611.
- ↑ "Intravenous mushroom poisoning". Ann Emerg Med 14 (9): 900–902. September 1985. doi:10.1016/s0196-0644(85)80643-0. PMID 4025991.
- ↑ "Hallucinogenic Drug Psilocybin Eases Existential Anxiety in People With Life-Threatening Cancer". Johns Hopkins. December 1, 2016. https://www.hopkinsmedicine.org/news/media/releases/hallucinogenic_drug_psilocybin_eases_existential_anxiety_in_people_with_life_threatening_cancer.
- ↑ "Psilocybin occasioned mystical-type experiences: immediate and persisting dose-related effects". Psychopharmacology 218 (4): 649–665. December 2011. doi:10.1007/s00213-011-2358-5. PMID 21674151.
- ↑ 40.0 40.1 "Natural sources of drugs of abuse: magic mushrooms". New Research on Street Drugs. New York, New York: Nova Science Publishers. 2006. pp. 167–186. ISBN 978-1-59454-961-8. https://books.google.com/books?id=ovGcMmz5emUC&pg=PA167. Retrieved February 27, 2016.
- ↑ 41.0 41.1 "Acute, subacute and long-term subjective effects of psilocybin in healthy humans: a pooled analysis of experimental studies". Journal of Psychopharmacology 25 (11): 1434–1452. November 2011. doi:10.1177/0269881110382466. PMID 20855349.
- ↑ "Experience of time and space in model psychoses". 50 Years of LSD. Current Status and Perspectives of Hallucinogens. New York, New York: The Parthenon Publishing Group. 1994. pp. 59–66. ISBN 978-1-85070-569-7.
- ↑ 43.0 43.1 "Effects of psilocybin on time perception and temporal control of behaviour in humans". Journal of Psychopharmacology 21 (1): 50–64. January 2007. doi:10.1177/0269881106065859. PMID 16714323.
- ↑ "Effects of varied doses of psilocybin on time interval reproduction in human subjects". Neuroscience Letters 435 (1): 51–55. April 2008. doi:10.1016/j.neulet.2008.02.006. PMID 18325673.
- ↑ "Using psilocybin to investigate the relationship between attention, working memory, and the serotonin 1A and 2A receptors". Journal of Cognitive Neuroscience 17 (10): 1497–1508. October 2005. doi:10.1162/089892905774597191. PMID 16269092. http://www.zora.uzh.ch/id/eprint/121457/1/089892905774597191.pdf. Retrieved August 16, 2019.
- ↑ "Neural underpinnings of temporal processing: a review of focal lesion, pharmacological, and functional imaging research". Reviews in the Neurosciences 10 (2): 91–116. 1999. doi:10.1515/REVNEURO.1999.10.2.91. PMID 10658954. https://escholarship.org/uc/item/0498n8nb.
- ↑ "Neuroanatomical and neurochemical substrates of timing". Neuropsychopharmacology 36 (1): 3–25. January 2011. doi:10.1038/npp.2010.113. PMID 20668434.
- ↑ 48.0 48.1 "[Automutilation after consumption of hallucinogenic mushrooms]" (in nl). Nederlands Tijdschrift voor Geneeskunde 151 (52): 2869–2872. December 2007. PMID 18257429.
- ↑ 49.0 49.1 49.2 Psychedelics Encyclopedia (3rd ed.). Berkeley, California: Ronin Publishing. 1992. ISBN 978-0-914171-51-5.
- ↑ 50.0 50.1 "Reactions to Psilocybjn Administered in a Supportive Environment". The Journal of Nervous and Mental Disease 137 (6): 561–573. December 1963. doi:10.1097/00005053-196312000-00007. PMID 14087676.
- ↑ "Breakdown or breakthrough? A history of European research into drugs and creativity". Journal of Creative Behavior 33 (4): 257–276. 1999. doi:10.1002/j.2162-6057.1999.tb01406.x. ISSN 0022-0175.
- ↑ "Modalities of the psychedelic experience: Microclimates of set and setting in hallucinogen research and culture". Transcultural Psychiatry 59 (5): 579–591. October 2022. doi:10.1177/13634615221100385. PMID 35818775.
- ↑ "Psychedelic Identity Shift: A Critical Approach to Set And Setting". Kennedy Institute of Ethics Journal 32 (4): 359–399. December 2022. doi:10.1353/ken.2022.0022. PMID 38588216.
- ↑ 54.0 54.1 54.2 "The relationship between the default mode network and the theory of mind network as revealed by psychedelics - A meta-analysis". Neuroscience and Biobehavioral Reviews 152. September 2023. doi:10.1016/j.neubiorev.2023.105325. PMID 37467907.
- ↑ "Theory of mind network activity is associated with metaethical judgment: An item analysis". Neuropsychologia 143. June 2020. doi:10.1016/j.neuropsychologia.2020.107475. PMID 32360298.
- ↑ "The default mode network and rumination in individuals at risk for depression". Social Cognitive and Affective Neuroscience 18 (1). June 2023. doi:10.1093/scan/nsad032. PMID 37261927.
- ↑ "Default Mode Network Modulation by Psychedelics: A Systematic Review". The International Journal of Neuropsychopharmacology 26 (3): 155–188. March 2023. doi:10.1093/ijnp/pyac074. PMID 36272145.
- ↑ "Drug Addictions, Hallucinogens and Shamanism: the View from Anthropology - Document - Gale Academic OneFile". https://go.gale.com/ps/i.do?id=GALE%7CA76445692&sid=googleScholar&v=2.1&it=r&linkaccess=abs&issn=15254283&p=AONE&sw=w&userGroupName=anon%7Ef134fbbe.
- ↑ 59.0 59.1 59.2 59.3 "Drug Addictions, Hallucinogens and Shamanism: the View from Anthropology". Drug Addictions, Hallucinogens and Shamanism. Townsend Letter for Doctors and Patients 217: 74–77. 2001. https://link.gale.com/apps/doc/A76445692/AONE?u=anon~f134fbbe&sid=googleScholar&xid=67117c36. Retrieved August 23, 2021.
- ↑ The psychedelic experience : a manual based on the Tibetan book of the dead. Ralph Metzner, Ram Dass, activeth century Karma-gliṅ-pa. New York: Citadel Press. 2007. ISBN 978-0-8065-1652-3. OCLC 318713242.
- ↑ "The Use of Classic Hallucinogens/Psychedelics in a Therapeutic Context: Healthcare Policy Opportunities and Challenges". Risk Management and Healthcare Policy 14: 901–910. 2024-04-24. doi:10.2147/RMHP.S300656. PMID 33707976.
- ↑ "Psilocybin-assisted group therapy: A new hope for demoralization". eClinicalMedicine 27. October 2020. doi:10.1016/j.eclinm.2020.100557. PMID 33073220.
- ↑ "Therapeutic bases of psychedelic medicines: psychointegrative effects". Psychedelic Medicine: New Evidence for Hallucinogenic Substances as Treatments. 1. Westport, Connecticut: Praeger. 2007. pp. 1–19. ISBN 978-0-275-99024-4. https://archive.org/details/psychedelicmedic0000unse/page/1.
- ↑ Food of the Gods: The Search for the Original Tree of Knowledge. A Radical History of Plants, Drugs, and Human Evolution. New York, New York: Bantam Books. 1993. ISBN 978-0-553-37130-7.
- ↑ The Varieties of Religious Experience. New York, New York: Simon & Schuster. 1997. ISBN 978-0-684-84297-4.
- ↑ "Hallucinogenic drugs and plants in psychotherapy and shamanism". Journal of Psychoactive Drugs 30 (4): 333–341. 1998. doi:10.1080/02791072.1998.10399709. PMID 9924839. http://cdn.preterhuman.net/texts/religion.occult.new_age/occult_library/Metzner_R-Hallucinogenic_Plants.pdf. Retrieved October 26, 2017.
- ↑ "Implications of LSD and experimental mysticism". Journal of Religion and Health 5 (3): 175–208. July 1966. doi:10.1007/BF01532646. PMID 24424798.
- ↑ "Drugs and mysticism". International Journal of Parapsychology 8 (2): 295–315. 1966.
- ↑ 69.0 69.1 "Mystical-type experiences occasioned by psilocybin mediate the attribution of personal meaning and spiritual significance 14 months later". Journal of Psychopharmacology 22 (6): 621–632. August 2008. doi:10.1177/0269881108094300. PMID 18593735. PMC 3050654. http://www.csp.org/psilocybin/Hopkins-CSP-Psilocybin2008.pdf. Retrieved July 3, 2008.
- ↑ Cleansing the Doors of Perception: The Religious Significance of Entheogenic Plants and Chemicals. New York, New York: Jeremy P. Tarcher/Putnam. 2000. p. 101. ISBN 978-1-58542-034-6. https://archive.org/details/cleansingdoorsof00hust/page/101.
- ↑ 71.0 71.1 "Pahnke's "Good Friday Experiment": a long-term follow-up and methodological critique". Journal of Transpersonal Psychology 23 (1): 1–25. 1991.
- ↑ "The phenomenology and potential religious import of states of consciousness facilitated by psilocybin". Archive for the Psychology of Religion 30 (1): 189–199. 2008. doi:10.1163/157361208X317196.
- ↑ "Psilocybin can occasion mystical-type experiences having substantial and sustained personal meaning and spiritual significance". Psychopharmacology 187 (3): 268–283. August 2006. doi:10.1007/s00213-006-0457-5. PMID 16826400. http://www.hopkinsmedicine.org/bin/s/m/GriffithsPsilocybin.pdf.
- ↑ "Press release: Griffiths psilocybin". Johns Hopkins Medicine. July 11, 2006. http://www.hopkinsmedicine.org/Press_releases/2006/GriffithspsilocybinQ.
- ↑ "The significance of finerenone as a novel therapeutic option in diabetic kidney disease: a scoping review with emphasis on cardiorenal outcomes of the finerenone phase 3 trials". Frontiers in Medicine 11 (1). 1975. doi:10.2307/1384454. PMID 38947237.
- ↑ "Medical News: Psilocybin Viewed as Therapy or Research Tool". July 12, 2006. http://www.medpagetoday.com/Psychiatry/GeneralPsychiatry/tb/3721.
- ↑ 77.0 77.1 "User perceptions of the benefits and harms of hallucinogenic drug use: a web-based questionnaire study". Journal of Substance Use 15 (4): 283–300. 2010. doi:10.3109/14659890903271624.
- ↑ 78.0 78.1 78.2 78.3 78.4 78.5 78.6 78.7 78.8 "The pharmacology of psilocybin". Addiction Biology 7 (4): 357–364. October 2002. doi:10.1080/1355621021000005937. PMID 14578010. https://citeseerx.ist.psu.edu/document?repid=rep1&type=pdf&doi=981b848a2785bc0c9b5a9afa1162c0c43b4d96c9. "An interesting fact may be the much shorter half-life (mean 74.1 ± 19.6 minutes i.v. compared to 163 ± 64 minutes p.o.) and duration of action (subjective effects lasting only 15–30 minutes) when psilocybin is given intravenously, as performed in a recent double-blind placebo controlled trial.29".
- ↑ Quentin AM (1960). La Psilocybine en Psychiatrie Clinique et Experimentale [Psilocybin in Clinical and Experimental Psychiatry] (PhD thesis) (in français). Paris, France: Paris University Medical Dissertation.
- ↑ See for example:
- "Comparison of the reactions induced by psilocybin and LSD-25 in man". Psychopharmacologia 1 (1): 29–38. 1959. doi:10.1007/BF00408109. PMID 14405870.
- "Comparison of three psychotropic drugs (psilocybin, JB-329, and IT-290) in volunteer subjects". The Journal of Nervous and Mental Disease 131 (5): 428–434. November 1960. doi:10.1097/00005053-196011000-00007. PMID 13715375.
- "Some observations on psilocybin, a new hallucinogen, in volunteer subjects". Comprehensive Psychiatry 1: 8–17. February 1960. doi:10.1016/S0010-440X(60)80045-4. PMID 14420328. * "Experimental psychiatry. V. Psilocybine, a new psychotogenic drug". The New England Journal of Medicine 262 (6): 295–297. February 1960. doi:10.1056/NEJM196002112620606. PMID 14437505.
- "The psilocybin-induced "state of drunkenness" in normal volunteers and schizophrenics". Behavioral Neuropsychiatry 8 (1–12): 83–86. 1976. PMID 1052267.
- ↑ 81.00 81.01 81.02 81.03 81.04 81.05 81.06 81.07 81.08 81.09 81.10 81.11 81.12 81.13 81.14 81.15 81.16 "Pharmacokinetics and Pharmacodynamics of Oral Psilocybin Administration in Healthy Participants". Clin Pharmacol Ther 113 (4): 822–831. April 2023. doi:10.1002/cpt.2821. PMID 36507738. https://www.researchgate.net/publication/366214647.
- ↑ 82.0 82.1 82.2 Cite error: Invalid
<ref>tag; no text was provided for refs namedHaslerBourquinBrenneisen1997 - ↑ "Psychedelics". Pharmacological Reviews 68 (2): 264–355. April 2016. doi:10.1124/pr.115.011478. PMID 26841800. "[...] psilocybin is not typically administered to humans by injection, nor are Psilocybe mushrooms generally taken in routes other than by mouth. In the Hermle et al. (1992), Vollenweider et al. (1997a,b), and GouzoulisMayfrank et al. (1999) studies cited earlier, the drug was administered orally, but psilocybin was administered intravenously in the studies by Carhart-Harris et al. Subjective reports from the two different routes of administration indicate profound differences in the speed of onset, as well as the intensity of the subjective effects. Psilocybin given orally generally takes about 40 minutes to begin to manifest its effect, with a duration of action lasting 4–6 hours. By contrast, subjective effects of 2 mg psilocybin given as an intravenous injection over 60 seconds begin at the injection period, reach a sustained peak after 4 minutes, and subside completely after 45–60 minutes (Carhart-Harris et al., 2011).".
- ↑ "The administration of psilocybin to healthy, hallucinogen-experienced volunteers in a mock-functional magnetic resonance imaging environment: a preliminary investigation of tolerability". Journal of Psychopharmacology 25 (11): 1562–1567. November 2011. doi:10.1177/0269881110367445. PMID 20395317.
- ↑ "Reconsidering evidence for psychedelic-induced psychosis: an overview of reviews, a systematic review, and meta-analysis of human studies". Mol Psychiatry 30 (3): 1223–1255. March 2025. doi:10.1038/s41380-024-02800-5. PMID 39592825.
- ↑ "A Plea for Nuance: Should People with a Family History of Bipolar Disorder Be Excluded from Clinical Trials of Psilocybin Therapy?". Psychedelic Med (New Rochelle) 2 (2): 61–73. June 2024. doi:10.1089/psymed.2023.0051. PMID 40051581. PMC 11658676. https://odonovanlab.ucsf.edu/sites/g/files/tkssra7476/f/Downey%2C%20A.%20E.%20-%20A%20Plea%20for%20Nuance_%20Should%20People%20with%20a%20Family%20History%20of%20Bipolar%20Disorder%20Be%20Excluded%20from%20Clinical%20Trials%20of%20Psilocybin%20Therapy_.pdf#page=2.
- ↑ 87.0 87.1 87.2 "Efficacy and safety of psychedelics for the treatment of mental disorders: A systematic review and meta-analysis". Psychiatry Res 335. May 2024. doi:10.1016/j.psychres.2024.115886. PMID 38574699.
- ↑ "Could psychedelic drugs have a role in the treatment of schizophrenia? Rationale and strategy for safe implementation". Mol Psychiatry 28 (1): 44–58. January 2023. doi:10.1038/s41380-022-01832-z. PMID 36280752.
- ↑ "Psychedelics and schizophrenia: a double-edged sword". Mol Psychiatry 30 (2): 679–692. February 2025. doi:10.1038/s41380-024-02743-x. PMID 39294303.
- ↑ "Psychedelics action and schizophrenia". Pharmacol Rep 75 (6): 1350–1361. December 2023. doi:10.1007/s43440-023-00546-5. PMID 37899392.
- ↑ "Psychedelics in the treatment of unipolar and bipolar depression". Int J Bipolar Disord 10 (1). July 2022. doi:10.1186/s40345-022-00265-5. PMID 35788817.
- ↑ "Exploring Serotonergic Psychedelics as a Treatment for Personality Disorders". Neuropharmacology 272. March 2025. doi:10.1016/j.neuropharm.2025.110413. PMID 40081794.
- ↑ 93.00 93.01 93.02 93.03 93.04 93.05 93.06 93.07 93.08 93.09 93.10 93.11 93.12 "Drug-drug interactions involving classic psychedelics: A systematic review". J Psychopharmacol 38 (1): 3–18. January 2024. doi:10.1177/02698811231211219. PMID 37982394.
- ↑ 94.0 94.1 94.2 94.3 94.4 94.5 "Trip-killers: a concerning practice associated with psychedelic drug use". Emerg Med J 41 (2): 112–113. January 2024. doi:10.1136/emermed-2023-213377. PMID 38123961. https://s3.amazonaws.com/crawl.prod.proquest.com/fpcache/71f445805bfb61341cbc438c8ae23bd3.pdf?X-Amz-Security-Token=IQoJb3JpZ2luX2VjEBMaCXVzLWVhc3QtMSJIMEYCIQDETX7YpaG5THA%2FNbKR0d92wr6h%2Bgg9preNcKjAsEqo%2BQIhAIlPGGWOeUc23LqhBzRYbxvSXB9aqSe2vVonl4nacAhhKp0CCLz%2F%2F%2F%2F%2F%2F%2F%2F%2F%2FwEQABoMNTE4MzQ2ODQ4MzQxIgy9ji58Qtbi%2BavuKeYq8QEDL1U5KZDQ0bXFyVapeqJgE%2FX6x8DcJfFU8DAXYZPSQEwrIdfPbZWcYsH340deru%2FUHnNaGGpuHFoVzui%2FMbqBz7MANcowj%2FL1%2BQZzQ5hXh5KM3BW8E6NRzrQyuPRmBy7kQUkx8%2BjTN%2BXSMgF%2FCAs6Dn9fScgBGz3ddkwRZXDkjasqMP65RCPKhagK68cyMbf3oX%2BKS8a4Kltc2rk3CnWEhOKrZU4mIxq07DikLAXQbl8YRZJIkeOhN5TgBaLWJqyn1td2VWCMymAaFsqtPWHwXnEfsolRlfDooe6QXfE2YwX5PxBVJU7GPXRgrAqPjwtJMOCHgsEGOpwBYif%2BaDMBdz3IEghuvCvorAS0mkHzdcOz%2Fi7AzuN9nch%2FIm8llhMsN41aAWHuSG25pnhhftauFsg7rbGsrW2nl2kq2upi9zP7y%2Fnqk93jcP0kr0jM8zU12bYoSTsToQJsshH4N%2BTQUMwlzRQfeVv8MXdq%2BgSTTzJrWNwT1yNzye3rSHjvOumbNl6sgBISw7QqRzhB6hZTuf8AcI%2B7&X-Amz-Algorithm=AWS4-HMAC-SHA256&X-Amz-Date=20250511T111826Z&X-Amz-SignedHeaders=host&X-Amz-Credential=ASIAXRL7BHBKRAKCQVVB%2F20250511%2Fus-east-1%2Fs3%2Faws4_request&X-Amz-Expires=3600&X-Amz-Signature=20bb1b90e4c8dbaa4115c954387617ebe2f55269bdd517692f1380193ed3f769.
- ↑ 95.0 95.1 95.2 "Psilocybin (Magic Mushrooms)". National Institute on Drug Abuse, US National Institutes of Health. 2024-01-24. https://nida.nih.gov/research-topics/psilocybin-magic-mushrooms.
- ↑ Handbook of Child and Adolescent Drug and Substance Abuse: Pharmacological, Developmental, and Clinical Considerations (2nd ed.). Hoboken, New Jersey: John Wiley & Sons. 2012. p. 199. ISBN 978-0-470-63906-1. https://books.google.com/books?id=Ox0U5nIZRQ8C&pg=PT257. Retrieved February 27, 2016.
- ↑ Drugs During Pregnancy and Lactation: Handbook of Prescription Drugs and Comparative Risk Assessment. Amsterdam, the Netherlands: Elsevier. 2001. p. 222. ISBN 978-0-444-50763-1. https://books.google.com/books?id=CE569saGK70C&pg=PA222. Retrieved February 27, 2016.
- ↑ "The problem of psilocybin mushroom abuse". Human Toxicology 1 (4): 417–424. October 1982. doi:10.1177/096032718200100408. PMID 7173927. Bibcode: 1982HETox...1..417P.
- ↑ 99.0 99.1 "Psilocybin induces schizophrenia-like psychosis in humans via a serotonin-2 agonist action". NeuroReport 9 (17): 3897–3902. December 1998. doi:10.1097/00001756-199812010-00024. PMID 9875725. http://pdfs.semanticscholar.org/b67b/b9f54c0e79bc73c8b205304a2f0d29eeb2a9.pdf.
- ↑ "Abuse of indigenous psilocybin mushrooms: a new fashion and some psychiatric complications". The British Journal of Psychiatry 132 (6): 602–604. June 1978. doi:10.1192/bjp.132.6.602. PMID 566144.
- ↑ "Phenomenally phunny phungi--psilocybin toxicity". North Carolina Medical Journal 44 (10): 639–640. October 1983. PMID 6580536.
- ↑ "Depersonalisation disorder: a contemporary overview". CNS Drugs 18 (6): 343–354. 2004. doi:10.2165/00023210-200418060-00002. PMID 15089102.
- ↑ "Khat and mushrooms associated with psychosis". The World Journal of Biological Psychiatry 5 (1): 49–53. January 2004. doi:10.1080/15622970410029908. PMID 15048636.
- ↑ "Behavioral studies of hallucinogenic drugs in animals: implications for schizophrenia research". Pharmacopsychiatry 31 (S2): 73–79. July 1998. doi:10.1055/s-2007-979350. PMID 9754837.
- ↑ 105.0 105.1 "A systems model of altered consciousness: integrating natural and drug-induced psychoses". Brain Research Bulletin 56 (5): 495–507. November 2001. doi:10.1016/S0361-9230(01)00646-3. PMID 11750795.
- ↑ "Serotonin research: contributions to understanding psychoses". Trends in Pharmacological Sciences 29 (9): 445–453. September 2008. doi:10.1016/j.tips.2008.06.006. PMID 19086254.
- ↑ "Flashbacks in theory and practice". The Heffter Review of Psychedelic Research 1: 51–57. 1998. http://www.heffter.org/docs/hrireview/01/chapter7.pdf. Retrieved August 14, 2011.
- ↑ "Acute toxicity of drugs versus regulatory status". Drugs and Society: U.S. Public Policy. Lanham, Maryland: Rowman & Littlefield. 2006. pp. 149–162; Table 7.1 "Safety Ratio and Dependence Potential of Psychoactive Drugs". ISBN 978-0-7425-4245-7. http://web.cgu.edu/faculty/gabler/drug_toxicity.htm.
- ↑ Psilocybin Mushroom Handbook: Easy Indoor and Outdoor Cultivation. Oakland, California: Quick American Archives. 2006. p. 164. ISBN 978-0-932551-71-9. https://books.google.com/books?id=HJJmJYCl3HsC&pg=PA164. Retrieved February 27, 2016.
- ↑ "The pharmacology of lysergic acid diethylamide: a review". CNS Neuroscience & Therapeutics 14 (4): 295–314. 2008. doi:10.1111/j.1755-5949.2008.00059.x. PMID 19040555.
- ↑ 111.0 111.1 111.2 111.3 111.4 111.5 111.6 111.7 "Multiple receptors contribute to the behavioral effects of indoleamine hallucinogens". Neuropharmacology 61 (3): 364–381. September 2011. doi:10.1016/j.neuropharm.2011.01.017. PMID 21256140.
- ↑ "Early-onset drug use and risk for drug dependence problems". Addictive Behaviors 34 (3): 319–322. March 2009. doi:10.1016/j.addbeh.2008.10.021. PMID 19022584.
- ↑ "Ranking the harm of alcohol, tobacco and illicit drugs for the individual and the population". European Addiction Research 16 (4): 202–207. 2010. doi:10.1159/000317249. PMID 20606445.
- ↑ "Drug harms in the UK: a multicriteria decision analysis". Lancet 376 (9752): 1558–1565. November 2010. doi:10.1016/S0140-6736(10)61462-6. PMID 21036393.
- ↑ 115.0 115.1 115.2 "The risk of chronic psychedelic and MDMA microdosing for valvular heart disease". J Psychopharmacol 37 (9): 876–890. September 2023. doi:10.1177/02698811231190865. PMID 37572027.
- ↑ 116.0 116.1 116.2 "Microdosing psychedelics and the risk of cardiac fibrosis and valvulopathy: Comparison to known cardiotoxins". J Psychopharmacol 38 (3): 217–224. March 2024. doi:10.1177/02698811231225609. PMID 38214279.
- ↑ 117.0 117.1 117.2 117.3 117.4 Toxicology and Pharmacological Interactions of Classic Psychedelics. Current Topics in Behavioral Neurosciences. Berlin, Heidelberg: Springer Berlin Heidelberg. 2024. doi:10.1007/7854_2024_508. https://link.springer.com/10.1007/7854_2024_508. Retrieved 14 May 2025.
- ↑ 118.0 118.1 "Management of overdoses of salvia, kratom, and psilocybin mushrooms: a literature review". Expert Review of Clinical Pharmacology 13 (8): 847–856. August 2020. doi:10.1080/17512433.2020.1794811. PMID 32648791.
- ↑ 119.0 119.1 "Serotonin toxicity of serotonergic psychedelics". Psychopharmacology (Berl) 239 (6): 1881–1891. June 2022. doi:10.1007/s00213-021-05876-x. PMID 34251464.
- ↑ 120.0 120.1 "Concomitant use of antidepressants and classic psychedelics: A scoping review". J Psychopharmacol: 2698811251368360. September 2025. doi:10.1177/02698811251368360. PMID 40937732. "Importantly, the idea of increased risk for developing serotonin syndrome and/or serotonin toxicity, when [antidepressants (ADs)] are co-administered with high doses of psychedelics, has recently been challenged, in part because classic psychedelics are partial agonists of the 5-HT2A receptor and would also compete for serotonin binding (Malcolm and Thomas, 2022; Rickli et al., 2016; Sarparast et al., 2022). Based on this clinical rationale, the concomitant use of ADs and classic psychedelics may be preferred, or patients could temporarily reduce the dose of ADs around dosing days with psychedelics to have minimal interactions. Simultaneously, the competition for 5-HT2A receptors could impede the biological action of psychedelics during concomitant use of ADs and potentially limit efficacy (Halman et al., 2024), particularly as the 5-HT2A receptor induces neuroplasticity (Cameron et al., 2023; Ly et al., 2018; Vargas et al., 2023).".
- ↑ Cite error: Invalid
<ref>tag; no text was provided for refs namedMerck - ↑ 122.0 122.1 The Merck Index: An Encyclopedia of Chemicals, Drugs, and Biologicals (13th ed.). Whitehouse Station, New Jersey: Merck. 2001. p. 1419. ISBN 978-0-911910-13-1. https://archive.org/details/merckindexency00onei/page/1419.
- ↑ "Comparison of acute lethal toxicity of commonly abused psychoactive substances". Addiction 99 (6): 686–696. June 2004. doi:10.1111/j.1360-0443.2004.00744.x. PMID 15139867. http://web.cgu.edu/faculty/gabler/toxicity%20Addiction%20offprint.pdf. Retrieved November 16, 2011.
- ↑ Inner Paths to Outer Space: Journeys to Alien Worlds through Psychedelics and Other Spiritual Technologies. Rochester, Vermont: Park Street Press. 2008. p. 147. ISBN 978-1-59477-224-5. https://books.google.com/books?id=0P3_kfFtgicC&pg=PT164. Retrieved February 27, 2016.
- ↑ "Intoxication mortelle à la suite de la consommation volontaire et en groupe de champignons hallucinogènes" (in fr). Bulletin de la Société Mycologique de France 112: 1–14. 1996.
- ↑ "On the presumed French case of fatality caused by ingestion of Liberty Caps". Eluesis 6: 40–41. 1996. http://www.museocivico.rovereto.tn.it/pubblicazioni.jsp?ID_LINK=111251&area=3.
- ↑ 127.00 127.01 127.02 127.03 127.04 127.05 127.06 127.07 127.08 127.09 127.10 127.11 "Drug-drug interactions between psychiatric medications and MDMA or psilocybin: a systematic review". Psychopharmacology (Berl) 239 (6): 1945–1976. June 2022. doi:10.1007/s00213-022-06083-y. PMID 35253070.
- ↑ 128.0 128.1 128.2 128.3 Jayasinha BG (8 February 2024). Towards Safer Trips: Exploring Harm Reduction Strategies for Recreational Psychedelic Use in Aotearoa New Zealand (Masters of Arts thesis). University of Otago. Retrieved 3 October 2024.
- ↑ 129.0 129.1 129.2 129.3 "Study Finds Hundreds of Reddit Posts on "Trip-Killers" for Psychedelic Drugs". JAMA 331 (8): 632–634. February 2024. doi:10.1001/jama.2023.28257. PMID 38294772.
- ↑ 130.0 130.1 "Return of the lysergamides. Part IV: Analytical and pharmacological characterization of lysergic acid morpholide (LSM-775)". Drug Test Anal 10 (2): 310–322. February 2018. doi:10.1002/dta.2222. PMID 28585392. "Additionally, pretreatment with the 5‐HT1A agonist buspirone (20 mg p.o.) markedly attenuates the visual effects of psilocybin in human volunteers.59 Although buspirone failed to completely block the hallucinogenic effects of psilocybin, the limited inhibition is not necessarily surprising because buspirone is a low efficacy 5‐HT1A partial agonist.60 The level of 5‐HT1A activation produced by buspirone may not be sufficient to completely counteract the stimulation of 5‐HT2A receptors by psilocin (the active metabolite of psilocybin). Another consideration is that psilocin acts as a 5‐HT1A agonist.30 If 5‐HT1A activation by psilocin buffers its hallucinogenic effects similar to DMT58 then competition between psilocin and a weaker partial agonist such as buspirone would limit attenuation of the hallucinogenic response.".
- ↑ 131.0 131.1 131.2 "Modulatory effect of the 5-HT1A agonist buspirone and the mixed non-hallucinogenic 5-HT1A/2A agonist ergotamine on psilocybin-induced psychedelic experience". Eur Neuropsychopharmacol 26 (4): 756–766. April 2016. doi:10.1016/j.euroneuro.2016.01.005. PMID 26875114.
- ↑ 132.0 132.1 "Human psychopharmacology of N,N-dimethyltryptamine". Behav Brain Res 73 (1–2): 121–124. 1996. doi:10.1016/0166-4328(96)00081-2. PMID 8788488.
- ↑ 133.0 133.1 "Acute Effects of Psilocybin After Escitalopram or Placebo Pretreatment in a Randomized, Double-Blind, Placebo-Controlled, Crossover Study in Healthy Subjects". Clin Pharmacol Ther 111 (4): 886–895. April 2022. doi:10.1002/cpt.2487. PMID 34743319.
- ↑ "GABAA receptor: Positive and negative allosteric modulators". Neuropharmacology 136 (Pt A): 10–22. July 2018. doi:10.1016/j.neuropharm.2018.01.036. PMID 29407219.
- ↑ "The Tolerability and Safety of Psilocybin in Psychiatric and Substance-Dependence Conditions: A Systematic Review". The Annals of Pharmacotherapy 58 (8): 811–826. August 2024. doi:10.1177/10600280231205645. PMID 37902038.
- ↑ "Clinical features and management of intoxication due to hallucinogenic drugs". Medical Toxicology and Adverse Drug Experience (Springer Science and Business Media LLC) 4 (5): 324–350. 1989. doi:10.1007/bf03259916. PMID 2682130.
- ↑ "Hallucinogens". Addiction Medicine. New York, NY: Springer New York. 2010. pp. 1083–1098. doi:10.1007/978-1-4419-0338-9_54. ISBN 978-1-4419-0337-2.
- ↑ "Psychedelic mushroom-containing chocolate exposures: Case series". The American Journal of Emergency Medicine 85: 208–213. November 2024. doi:10.1016/j.ajem.2024.09.038. PMID 39288500.
- ↑ "Psychedelic research, assisted therapy and the role of the anaesthetist: A review and insights for experimental and clinical practices". British Journal of Clinical Pharmacology 90 (12): 3119–3134. December 2024. doi:10.1111/bcp.16264. PMID 39380091.
- ↑ "Co-administration of midazolam and psilocybin: differential effects on subjective quality versus memory of the psychedelic experience". Translational Psychiatry 14 (1). September 2024. doi:10.1038/s41398-024-03059-8. PMID 39266503.
- ↑ "Practical considerations in the establishment of psychedelic research programs". Psychopharmacology 242 (1): 27–43. January 2025. doi:10.1007/s00213-024-06722-6. PMID 39627438. "Furthermore, benzodiazepines might attenuate the antidepressant effects of psychedelics (Hibicke et al. 2024).".
- ↑ "Preadministration of Lorazepam Reduces Efficacy and Longevity of Antidepressant-Like Effect from a Psychedelic". Psychedelic Medicine 2 (1): 10–14. March 2024. doi:10.1089/psymed.2023.0037. PMID 40051761.
- ↑ 143.00 143.01 143.02 143.03 143.04 143.05 143.06 143.07 143.08 143.09 143.10 143.11 143.12 143.13 143.14 143.15 143.16 143.17 143.18 143.19 143.20 143.21 143.22 143.23 "In vitro and in vivo metabolism of psilocybin's active metabolite psilocin". Front Pharmacol 15. 2024. doi:10.3389/fphar.2024.1391689. PMID 38741590.
- ↑ "Monoamine oxidase inhibition in cigarette smokers: From preclinical studies to tobacco product regulation". Front Neurosci 16. 2022. doi:10.3389/fnins.2022.886496. PMID 36051642.
- ↑ "Hypertensive Emergency Secondary to Combining Psilocybin Mushrooms, Extended Release Dextroamphetamine-Amphetamine, and Tranylcypromine". J Psychoactive Drugs 57 (3): 297–303. June 2024. doi:10.1080/02791072.2024.2368617. PMID 38903003.
- ↑ "Ovlvinĕní experimntálních psychóz po psilocybinu inhibitory MAO" (in Czech). Act Nerv Super (Praha) 10 (3): 278–279. October 1968. PMID 5702524.
- ↑ "Higher affinity of psilocin for human than rat 5-HT2 receptor indicates binding site structure". Medicinal Chemistry Research 3: 52–66. 1993. https://scholar.google.com/scholar?cluster=2484757771892655822.
- ↑ Cite error: Invalid
<ref>tag; no text was provided for refs namedRothmanPartillaBaumann2012 - ↑ Cite error: Invalid
<ref>tag; no text was provided for refs namedBloughLandavazoDecker2014 - ↑ 150.0 150.1 Cite error: Invalid
<ref>tag; no text was provided for refs namedRickliMoningHoener2016 - ↑ Cite error: Invalid
<ref>tag; no text was provided for refs namedBindingDB-Psilocin - ↑ Cite error: Invalid
<ref>tag; no text was provided for refs namedPDSP-Psilocin - ↑ Cite error: Invalid
<ref>tag; no text was provided for refs namedGainetdinovHoenerBerry2018 - ↑ 154.0 154.1 "Role of 5-HT2A, 5-HT2C, 5-HT1A and TAAR1 Receptors in the Head Twitch Response Induced by 5-Hydroxytryptophan and Psilocybin: Translational Implications". Int J Mol Sci 23 (22). November 2022. doi:10.3390/ijms232214148. PMID 36430623.
- ↑ "Serotonin 5-HT2A, 5-HT2c and 5-HT1A receptor involvement in the acute effects of psilocybin in mice. In vitro pharmacological profile and modulation of thermoregulation and head-twich response". Biomed Pharmacother 154. October 2022. doi:10.1016/j.biopha.2022.113612. PMID 36049313.
- ↑ "Psychedelic effects of psilocybin correlate with serotonin 2A receptor occupancy and plasma psilocin levels". Neuropsychopharmacology 44 (7): 1328–1334. June 2019. doi:10.1038/s41386-019-0324-9. PMID 30685771.
- ↑ "Serotonergic Psychedelics: Experimental Approaches for Assessing Mechanisms of Action". Handb Exp Pharmacol. Handbook of Experimental Pharmacology 252: 227–260. 2018. doi:10.1007/164_2018_107. ISBN 978-3-030-10560-0. PMID 29532180. "Reports from clinical trials conclude that the psychedelic effects of psilocybin and LSD are mediated by 5-HT2A receptors, because they are blocked by ketanserin (40 mg, P.O.), typically viewed as a selective 5-HT2A antagonist (Kometer et al. 2012; Kraehenmann et al. 2017; Preller et al. 2017; Quednow et al. 2012). Haloperidol, typically viewed as a selective dopamine D2 antagonist, is much less effective than ketanserin at blocking psilocybin's effects, but risperidone, an antipsychotic with combined D2/5-HT2 activity, is as effective as ketanserin (Vollenweider et al. 1998).".
- ↑ "D-Lysergic acid diethylamide, psilocybin, and other classic hallucinogens: Mechanism of action and potential therapeutic applications in mood disorders". Psychedelic Neuroscience. Progress in Brain Research. 242. 2018. pp. 69–96. doi:10.1016/bs.pbr.2018.07.008. ISBN 978-0-12-814255-4. "Noteworthy, the activation of postsynaptic 5HT2A receptor in layer V of the medial prefrontal cortex (mPFC) is considered to be responsible for the visual hallucinations produced by LSD and other psychedelic drugs such as psilocybin (Jakab and Goldman-Rakic, 1998; Vollenweider and Kometer, 2010) (see Fig. 2). [...] Although the classic hallucinogens LSD and psilocybin do not have a direct affinity for glutamate receptors, several animal studies have highlighted that glutamate carries a significant weight of the overall downstream effects of LSD and hallucinogenic action. The activation of postsynaptic cortical 5HT2A increases extracellular glutamate release in the synaptic cleft which is reversed by selective 5-HT2A antagonists (Vollenweider et al., 1998), AMPA (α-amino-3-hydroxyl-5-methyl4-isoxazole-propionic acid) receptor antagonists (Zhang and Marek, 2008), agonists and positive allosteric modulators of mGluR2 (metabotropic glutamate receptor 2) (Benneyworth et al., 2007), and selective antagonists of the NR2B subunit of NMDA (N-methyl-D-aspartate) receptors (Lambe and Aghajanian, 2006). In particular, microdialysis in rats confirmed that systemic hallucinogen administration leads to a time-dependent increase in prefrontal cortex (PFC) glutamate levels, an effect which is blocked by administration with the selective 5HT2A antagonist M100907 (Muschamp et al., 2004)."
- ↑ "Cortical influences of serotonin and glutamate on layer V pyramidal neurons". 5-HT Interaction with Other Neurotransmitters: Experimental Evidence and Therapeutic Relevance - Part B. Progress in Brain Research. 261. 2021. pp. 341–378. doi:10.1016/bs.pbr.2020.11.002. ISBN 978-0-444-64258-5.
- ↑ 160.0 160.1 160.2 160.3 160.4 "Recent advances in the neuropsychopharmacology of serotonergic hallucinogens". Behav Brain Res 277: 99–120. January 2015. doi:10.1016/j.bbr.2014.07.016. PMID 25036425.
- ↑ 161.0 161.1 "Effect of Hallucinogens on Unconditioned Behavior". Behavioral Neurobiology of Psychedelic Drugs. Current Topics in Behavioral Neurosciences. 36. 2018. pp. 159–199. doi:10.1007/7854_2016_466. ISBN 978-3-662-55878-2. http://www.ouramazingworld.org/uploads/4/3/8/6/43860587/halberstadt2016.pdf. Retrieved February 7, 2025. "Compared with phenylalkylamines, tryptamine hallucinogens produce a disparate profile of effects in the mouse BPM. Administration of psilocin or 5-MeO-DMT produces a profound suppression of locomotor activity, investigatory holepokes and rearings, and center duration in C57BL/6J mice (Halberstadt et al. 2011). Most of these effects are blocked by pretreatment with the 5-HT1A antagonist WAY-100635, whereas the 5-HT2C antagonist SB242084 is ineffective."
- ↑ "Direct injection of 5-HT2A receptor agonists into the medial prefrontal cortex produces a head-twitch response in rats". J Pharmacol Exp Ther 282 (2): 699–706. August 1997. doi:10.1016/S0022-3565(24)36840-5. PMID 9262333.
- ↑ "Me, myself, bye: regional alterations in glutamate and the experience of ego dissolution with psilocybin". Neuropsychopharmacology 45 (12): 2003–2011. November 2020. doi:10.1038/s41386-020-0718-8. PMID 32446245.
- ↑ "The structural diversity of psychedelic drug actions revealed". Nature Communications 16 (1). March 2025. doi:10.1038/s41467-025-57956-7. PMID 40108183. Bibcode: 2025NatCo..16.2734G.
- ↑ "Structures of Hallucinogenic and Non-Hallucinogenic Analogues of the 5-HT2A Receptor Reveals Molecular Insights into Signaling Bias". University of North Carolina at Chapel Hill Department of Pharmacology Research Retreat September 16th, 2022 – William and Ida Friday Center. September 2022. https://www.med.unc.edu/pharm/wp-content/uploads/sites/930/2022/07/COMPLETE-PHARM-RETREAT-PROGRAM-2022-UPDATE.pdf#page=37.
- ↑ "Beyond the 5-HT2A Receptor: Classic and Nonclassic Targets in Psychedelic Drug Action". J Neurosci 43 (45): 7472–7482. November 2023. doi:10.1523/JNEUROSCI.1384-23.2023. PMID 37940583.
- ↑ 167.0 167.1 167.2 167.3 167.4 "ACNP 61st Annual Meeting: Poster Abstracts P271-P540: P314. Psilocybin Alters Behavior and the Intestinal Microbiota in a Wild Type Mouse Model by Mechanisms That Are Not Fully Dependent on 5HT2A and 5HT2C Receptors". Neuropsychopharmacology 47 (Suppl 1): 220–370 (245–246). December 2022. doi:10.1038/s41386-022-01485-0. PMID 36456694. "Psilocybin induced a robust head twitch response, increased exploratory behavior in the elevated plus maze, increased social behavior in the social interaction test, and decreased immobility in the forced swim test. Co-administration of ketanserin fully blocked the head twitch response without significantly altering psilocybin’s effects on other behavioral outcomes. In a separate cohort, treatment with psilocybin produced broad alteration of the intestinal microbiome, with particularly marked changes in the large intestine that were only partially blocked by pre-treatment with ketanserin. Finally, transplantation of intestinal contents from psilocybin-treated mice to naive untreated mice resulted in behavioral changes consistent with the effects of psilocybin treatment. [...] Our findings demonstrate that a single dose of psilocybin leads to behavioral changes in mice that are relevant for studies of resilience and affective disorders. Our results further indicate that the behavioral changes may not be fully dependent on psilocybin’s agonism of 5HT2A and 5HT2C receptors. Further, psilocybin appears to broadly alter the intestinal microbiome and transplantation of intestinal contents reproduces behavioral change associated with psilocybin treatment, suggesting a previously unknown microbiome-gut-brain mechanism of action.".
- ↑ "ACNP 61st Annual Meeting: Poster Abstracts P271-P540: P426. Translational Implications of Marble Burying in ICR Mice for the Anti-Obsessional Effects of Psilocybin". Neuropsychopharmacology 47 (Suppl 1): 220–370 (309–309). December 2022. doi:10.1038/s41386-022-01485-0. PMID 36456694.
- ↑ "Differential contributions of serotonin receptors to the behavioral effects of indoleamine hallucinogens in mice". J Psychopharmacol 25 (11): 1548–1561. November 2011. doi:10.1177/0269881110388326. PMID 21148021.
- ↑ "ACNP 63rd Annual Meeting: Poster Abstracts P609-P914: P691. The Non-Hallucinogenic Serotonin 1B Receptor is Necessary for the Persisting Behavioral Effects of Psilocybin in Mice". Neuropsychopharmacology 49 (Suppl 1): 418–594 (466). December 2024. doi:10.1038/s41386-024-02013-y. PMID 39643635.
- ↑ "The serotonin 5-HT2C receptor and the non-addictive nature of classic hallucinogens". J Psychopharmacol 31 (1): 127–143. January 2017. doi:10.1177/0269881116677104. PMID 27903793.
- ↑ 172.0 172.1 172.2 172.3 172.4 "The Effects of Psychedelics on Neuronal Physiology". Annu Rev Physiol 86: 27–47. February 2024. doi:10.1146/annurev-physiol-042022-020923. PMID 37931171.
- ↑ 173.0 173.1 "Psychedelics Promote Structural and Functional Neural Plasticity". Cell Rep 23 (11): 3170–3182. June 2018. doi:10.1016/j.celrep.2018.05.022. PMID 29898390.
- ↑ "Psychedelics promote neuroplasticity through the activation of intracellular 5-HT2A receptors". Science 379 (6633): 700–706. February 2023. doi:10.1126/science.adf0435. PMID 36795823. Bibcode: 2023Sci...379..700V.
- ↑ 175.0 175.1 "Psychedelics and Other Psychoplastogens for Treating Mental Illness". Frontiers in Psychiatry 12. 2021. doi:10.3389/fpsyt.2021.727117. PMID 34671279.
- ↑ 176.0 176.1 "Psychoplastogens: A Promising Class of Plasticity-Promoting Neurotherapeutics". Journal of Experimental Neuroscience 12. 2018-09-19. doi:10.1177/1179069518800508. PMID 30262987.
- ↑ 177.0 177.1 "Psychedelics and Neuroplasticity: A Systematic Review Unraveling the Biological Underpinnings of Psychedelics". Frontiers in Psychiatry 12. 2021. doi:10.3389/fpsyt.2021.724606. PMID 34566723.
- ↑ "Towards an understanding of psychedelic-induced neuroplasticity". Neuropsychopharmacology 48 (1): 104–112. January 2023. doi:10.1038/s41386-022-01389-z. PMID 36123427.
- ↑ "Psychedelics promote plasticity by directly binding to BDNF receptor TrkB". Nat Neurosci 26 (6): 1032–1041. June 2023. doi:10.1038/s41593-023-01316-5. PMID 37280397.
- ↑ "The polypharmacology of psychedelics reveals multiple targets for potential therapeutics". Neuron 113 (19): 3129–3142.e9. July 2025. doi:10.1016/j.neuron.2025.06.012. PMID 40683247. https://www.cell.com/cms/10.1016/j.neuron.2025.06.012/attachment/7d8365fe-51f3-4a28-bf40-9999bec837f6/mmc11.pdf. "Recent studies have suggested that psychedelics such as LSD directly interact with TrkB with high affinity, promoting BDNF-mediated neuroplasticity and antidepressant-like effects via allosteric potentiation of BDNF signaling in active synapses.8 To investigate this, we screened LSD across 450 human kinases, including TrkB, but found no significant interactions between LSD and any tested human kinases. Further experiments in transfected cells revealed no effect of LSD or psilocin on BDNF-mediated activation of a TrkB reporter. We note that similar negative preliminary results, which have not yet been published in a peer-reviewed journal, were recently reported by Boltaev et al.63".
- ↑ 181.0 181.1 181.2 "Psychedelics and Anti-inflammatory Activity in Animal Models". Disruptive Psychopharmacology. Current Topics in Behavioral Neurosciences. 56. 2022. pp. 229–245. doi:10.1007/7854_2022_367. ISBN 978-3-031-12183-8. "In our rodent acute asthma model, psilocin, the active metabolite of psilocybin, displays a similar anti-AHR efficacy and potency to that of (R)-DOI. Surprisingly, other tryptamines with very similar structures like N,N-dimethyltryptamine (DMT) and 5-methoxy-N,N-dimethyltryptamine (5-MeO-DMT) show no efficacy to reduce OVA-induced AHR."
- ↑ 182.0 182.1 182.2 "Preclinical perspectives on the mechanisms underlying the therapeutic actions of psilocybin in psychiatric disorders". Neuropharmacology 231. June 2023. doi:10.1016/j.neuropharm.2023.109504. PMID 36921889. "Interestingly, the anti-inflammatory effects of psychedelics acting at 5-HT2A receptors do not correlate with activation of either Gαq or β-arrestin recruitment (Flanagan et al., 2020), indicating psychedelics can recruit different effector pathways from those underlying behaviors for biological effects. [...] Psilocybin and certain other psychedelics have potent anti-inflammatory effects in preclinical models of human disease that could contribute to their efficacy. For example, delivery of psilocin directly to the lungs of rats via nebulization potently suppressed inflammation and restored normal breathing in a model of allergic asthma (Flanagan et al., 2020). The amount of psilocybin necessary for full effect was far below the threshold to produce behavioral effects, suggesting that sub-behavioral levels of psilocybin or other psychedelic may represent a new therapeutic strategy to treat inflammatory disorders. Interestingly, as mentioned above, neither the Gαq or β-arrestin signaling pathways seem to be involved in these effects (Flanagan et al., 2020).".
- ↑ 183.0 183.1 "Mushrooms, Microdosing, and Mental Illness: The Effect of Psilocybin on Neurotransmitters, Neuroinflammation, and Neuroplasticity". Neuropsychiatr Dis Treat 21: 141–155. 2025. doi:10.2147/NDT.S500337. PMID 39897712.
- ↑ "Psychedelics as potent anti-inflammatory therapeutics". Neuropharmacology 219. November 2022. doi:10.1016/j.neuropharm.2022.109232. PMID 36007854. "Remarkably, the IC50 dose for (R)-DOI in this prophylactic paradigm is ∼0.005 mg/kg, administered via nebulization or by intraperitoneal injection (Flanagan et al., 2021). This is > 50x less than the behavioral threshold dose. We have also shown that the drug psilocin, the active form of the prodrug psilocybin, has virtually the same potency as (R)-DOI (Flanagan et al., 2021), indicating that the effects are not limited to (R)-DOI or are chemotype dependent.".
- ↑ "Structure-Activity Relationship Analysis of Psychedelics in a Rat Model of Asthma Reveals the Anti-Inflammatory Pharmacophore". ACS Pharmacol Transl Sci 4 (2): 488–502. April 2021. doi:10.1021/acsptsci.0c00063. PMID 33860179.
- ↑ "Psychedelics as anti-inflammatory agents". Int Rev Psychiatry 30 (4): 363–375. August 2018. doi:10.1080/09540261.2018.1481827. PMID 30102081. http://usdbiology.com/cliff/Courses/Advanced%20Seminars%20in%20Neuroendocrinology/Therapeutic%20Effects%20of%20Psychedelics%2019/Flanagan%20Nichols%2018%20IntRevPsychiatry%20Psychedelics%20as%20anti-inflammatory%20agents.pdf. "We have previously speculated that the anti-inflammatory effects of psychedelics mediated through serotonin 5-HT2A receptor activation are a key component of not only the anti-depressant effects of psilocybin, but also contribute to its long-lasting effects after only a single treatment (Kyzar, Nichols, Gainetdinov, Nichols, & Kalueff, 2017).".
- ↑ "Microdosing psychedelics: More questions than answers? An overview and suggestions for future research". J Psychopharmacol 33 (9): 1039–1057. September 2019. doi:10.1177/0269881119857204. PMID 31303095.
- ↑ "The immunomodulatory effects of classical psychedelics: A systematic review of preclinical studies". Prog Neuropsychopharmacol Biol Psychiatry 136. September 2024. doi:10.1016/j.pnpbp.2024.111139. PMID 39251080.
- ↑ "Seeking the Psilocybiome: Psychedelics meet the microbiota-gut-brain axis". Int J Clin Health Psychol 23 (2). 2023. doi:10.1016/j.ijchp.2022.100349. PMID 36605409.
- ↑ "Psychedelics in the treatment of eating disorders: Rationale and potential mechanisms". Eur Neuropsychopharmacol 75: 1–14. October 2023. doi:10.1016/j.euroneuro.2023.05.008. PMID 37352816. "Interestingly, both EDs and mood disorders are often comorbid with gastrointestinal symptoms and reduced diversity of the gut microbiome. (Lam et al., 2017) A dysregulated microbiome may constitute a development or maintenance factor for AN in particular. (Butler et al., 2021) It has been suggested that psychedelics exert some of their long-term effects via the microbiome. (Kuypers, 2019) Psilocybin has been shown to diversify the intestinal microbiome in mice, and this diversification appeared to be responsible for lasting antidepressant-like behavioral effects. (Cordner et al., 2022) Normalization of the gut microbiome may thus assist with recovery from both EDs and comorbid mood disorders, and presents an intriguing avenue for future research. (Kleiman et al., 2015)".
- ↑ 191.0 191.1 "Do the therapeutic effects of psilocybin involve actions in the gut?". Trends Pharmacol Sci 45 (2): 107–117. February 2024. doi:10.1016/j.tips.2023.12.007. PMID 38216431.
- ↑ "Effect of oral tryptamines on the gut microbiome of rats-a preliminary study". PeerJ 12. 2024. doi:10.7717/peerj.17517. PMID 38846751.
- ↑ 193.0 193.1 "Serotonergic psychedelics as potential therapeutics for post-COVID-19 syndrome (or Long COVID): A comprehensive review". Prog Neuropsychopharmacol Biol Psychiatry 137. February 2025. doi:10.1016/j.pnpbp.2025.111279. PMID 39909170.
- ↑ 194.0 194.1 Cite error: Invalid
<ref>tag; no text was provided for refs namedWsół2023 - ↑ 195.0 195.1 "Effects of hallucinogenic drugs on the human heart". Front Pharmacol 15. 2024. doi:10.3389/fphar.2024.1334218. PMID 38370480.
- ↑ "Comparative acute effects of mescaline, lysergic acid diethylamide, and psilocybin in a randomized, double-blind, placebo-controlled cross-over study in healthy participants". Neuropsychopharmacology 48 (11): 1659–1667. October 2023. doi:10.1038/s41386-023-01607-2. PMID 37231080.
- ↑ 197.0 197.1 197.2 "Hallucinogens". Pharmacol Ther 101 (2): 131–181. February 2004. doi:10.1016/j.pharmthera.2003.11.002. PMID 14761703.
- ↑ 198.00 198.01 198.02 198.03 198.04 198.05 198.06 198.07 198.08 198.09 198.10 198.11 198.12 198.13 198.14 198.15 198.16 198.17 198.18 198.19 "Clinical Pharmacokinetics of Psilocin After Psilocybin Administration: A Systematic Review and Post-Hoc Analysis". Clin Pharmacokinet 64 (1): 53–66. January 2025. doi:10.1007/s40262-024-01454-4. PMID 39812743.
- ↑ 199.0 199.1 199.2 199.3 199.4 "Exploring Psychedelics Pharmacology: A Scoping Review Charting the Course of Psilocybin Pharmacokinetics". Clin Neuropharmacol 48 (1): 13–19. 2025. doi:10.1097/WNF.0000000000000617. PMID 39787428.
- ↑ "Pharmacokinetics of Escalating Doses of Oral Psilocybin in Healthy Adults". Clin Pharmacokinet 56 (12): 1543–1554. December 2017. doi:10.1007/s40262-017-0540-6. PMID 28353056.
- ↑ Cite error: Invalid
<ref>tag; no text was provided for refs namedČamparaKovačić2024 - ↑ 202.0 202.1 202.2 202.3 202.4 202.5 202.6 "Chemistry/structural biology of psychedelic drugs and their receptor(s)". Br J Pharmacol. October 2024. doi:10.1111/bph.17361. PMID 39354889. "In contrast to DMT, psilocybin is orally active. [...] A structurally related molecule, [5-HO-DMT], known as bufotenine, is inactive after oral administration. How does the simple transposition of the hydroxy from the 4 to the 5 position alter the physicochemical properties of the DMT core? We asked that question more than four decades ago. In a study by Migliaccio et al. (1981), the 360 MHz proton NMR, the amine pKa values and the octanol–water Log P values were determined experimentally and compared for both psilocin and bufotenine (Figure 3a). The side chain protons in the NMR for bufotenine were shown to be rapidly rotating with no preference for gauche or trans conformations, whereas the side chain for psilocin was less mobile and was determined to favour a gauche (80%) versus trans (20%) conformation. Because psilocin is a weaker base but is also more lipid soluble, it was proposed that psilocin formed an intramolecular hydrogen bond, as illustrated in Figure 3a. In the energy-minimized structure of this conformation, the length of the hydrogen bond is 1.88 Å. The weaker pKa of psilocin relative to bufotenine means that psilocin is less highly ionized at pH 7.4—that is, 8.5% free base versus 0.53% for bufotenine at pH 7.4. Ionized amines must be unionized and desolvated to cross the blood–brain barrier; the intramolecular H bond in psilocin compensates for that as reflected by the higher lipophilicity of psilocin relative to bufotenine. Finally, the mechanism of deamination by MAO involves either a single electron transfer or a nucleophilic mechanism (Gaweska & Fitzpatrick, 2011; Zapata-Torres et al., 2015), either of which is more enzymically difficult when the amine electrons are hydrogen-bonded by the 4-hydroxy group (Figure 3a). Very recently, Lenz et al. (2022) have confirmed and extended the finding of the potential intramolecular hydrogen bond partially being responsible for slow MAO deamination as well as psilocin's enhanced ability to cross the blood–brain barrier. [...] This would explain why bufotenine is still an agonist at the 5-HT2A receptor but due to its poor physiochemical properties is not psychoactive in humans.".
- ↑ 203.0 203.1 203.2 203.3 203.4 203.5 203.6 "Bufotenine: toward an understanding of possible psychoactive mechanisms". J Psychoactive Drugs 32 (3): 321–331. 2000. doi:10.1080/02791072.2000.10400456. PMID 11061684. https://citeseerx.ist.psu.edu/document?repid=rep1&type=pdf&doi=802678b9f472ee113a9df8011e68672c3681623d.
- ↑ 204.0 204.1 204.2 204.3 "Indole Alkaloids from Psychoactive Mushrooms: Chemical and Pharmacological Potential as Psychotherapeutic Agents". Biomedicines 11 (2): 461. February 2023. doi:10.3390/biomedicines11020461. PMID 36830997.
- ↑ 205.0 205.1 205.2 "Assessment of Bioactivity-Modulating Pseudo-Ring Formation in Psilocin and Related Tryptamines". ChemBioChem 23 (13). July 2022. doi:10.1002/cbic.202200183. PMID 35483009.
- ↑ "Psychedelic 5-methoxy-N,N-dimethyltryptamine: metabolism, pharmacokinetics, drug interactions, and pharmacological actions". Curr Drug Metab 11 (8): 659–666. October 2010. doi:10.2174/138920010794233495. PMID 20942780.
- ↑ Jonathan Ott (2001). "Pharmañopo-psychonautics: human intranasal, sublingual, intrarectal, pulmonary and oral pharmacology of bufotenine". Journal of Psychoactive Drugs 33 (3): 273–281. doi:10.1080/02791072.2001.10400574. PMID 11718320.
- ↑ 208.0 208.1 208.2 208.3 208.4 "Metabolism of psilocybin and psilocin: clinical and forensic toxicological relevance". Drug Metab Rev 49 (1): 84–91. February 2017. doi:10.1080/03602532.2016.1278228. PMID 28074670.
- ↑ 209.0 209.1 "Psilocybin containing mushrooms: a rapidly developing biotechnology industry in the psychiatry, biomedical and nutraceutical fields". 3 Biotech 12 (12). December 2022. doi:10.1007/s13205-022-03355-4. PMID 36340802.
- ↑ "Some biochemical studies on psilocybin and psilocin". Journal of Neuropsychiatry 4: 270–273. 1963. PMID 13954906. https://apps.dtic.mil/sti/tr/pdf/AD0291057.pdf#page=3.
- ↑ 211.0 211.1 "Elucidating the Phase I metabolism of psilocin in vitro". Arch Toxicol 99 (3): 1085–1094. January 2025. doi:10.1007/s00204-024-03952-7. PMID 39751877. Bibcode: 2025ArTox..99.1085C.
- ↑ "Indolealkylamines and Related Compounds". Hallucinogenic Agents. Bristol: Wright-Scientechnica. 1975. pp. 98–144. ISBN 978-0-85608-011-1. OCLC 2176880. https://bitnest.netfirms.com/external/Books/978-0-85608-011-1. Retrieved June 15, 2025. "The similarity of the pharmacological profiles of the two compounds coupled with demonstration of the in vivo dephosphorylation of psilocybin make it likely that the central effects of psilocybin are exerted only after its conversion into psilocin (Horita and Weber, 1962; Horita, 1963). Thus, administration of psilocybin to intact mice results in the accumulation of high concentrations of psilocin in the kidney and liver within 10-20 minutes and in the brain within 25-30 minutes, a distribution pattern identical to that observed following administration of psilocin itself (Kalberer, Kreis, and Rutschmann, 1962; Hopf and Eckert, 1969)."
- ↑ "Evolution of serotonin: sunlight to suicide". Handbook of the Behavioral Neurobiology of Serotonin. London, UK: Academic Press. 2010. p. 7. ISBN 978-0-12-374634-4. https://books.google.com/books?id=aomaKqIE1jUC&pg=PA7. Retrieved February 27, 2016.
- ↑ 214.0 214.1 214.2 214.3 "Psychoactive tryptamines from basidiomycetes". Folia Microbiologica 47 (1): 3–27. 2002. doi:10.1007/BF02818560. PMID 11980266.
- ↑ 215.0 215.1 "Psilocybine". U.S. National Library of Medicine. http://toxnet.nlm.nih.gov/cgi-bin/sis/search/r?dbs+hsdb:@term+@rn+@rel+520-52-5.
- ↑ "Psilocybin: crystal structure solutions enable phase analysis of prior art and recently patented examples". Acta Crystallographica, Section C 78 (Pt 1): 36–55. January 2022. doi:10.1107/S2053229621013164. PMID 34982048. Bibcode: 2022AcCrC..78...36S.
- ↑ 217.0 217.1 "Investigation into the temporal stability of aqueous standard solutions of psilocin and psilocybin using high performance liquid chromatography". Science & Justice 46 (2): 91–96. 2006. doi:10.1016/S1355-0306(06)71579-9. PMID 17002211.
- ↑ 218.0 218.1 "A Review of Synthetic Access to Therapeutic Compounds Extracted from Psilocybe". Pharmaceuticals 16 (1): 40. December 2022. doi:10.3390/ph16010040. PMID 36678537.
- ↑ "Hallucinogens". Principles of Forensic Toxicology (2nd ed.). Washington, D.C.: American Association for Clinical Chemistry Press. 2003. p. 281. ISBN 978-1-890883-87-4. https://books.google.com/books?id=k7BInEQ-iqgC&pg=PA281. Retrieved February 27, 2016.
- ↑ The Analysis of Controlled Substances. New York, Chichester: John Wiley and Sons. 2003. pp. 132–133. ISBN 978-0-471-49252-8. https://archive.org/details/analysisofcontro0000cole/page/132.
- ↑ A Colour Atlas of Poisonous Fungi: A Handbook for Pharmacists, Doctors, and Biologists. London, UK: Manson Publishing. 1989. p. 113. ISBN 978-0-7234-1576-3. https://books.google.com/books?id=EIcQGsZ2kksC&pg=PA113. Retrieved February 27, 2016.
- ↑ "Metabolism and toxicological analyses of hallucinogenic tryptamine analogues being abused in Japan". Forensic Toxicology 28 (1): 1–8. 2010. doi:10.1007/s11419-009-0087-9.
- ↑ 223.0 223.1 "Analysis of psilocybin and psilocin in Psilocybe subcubensis Guzmán by ion mobility spectrometry and gas chromatography-mass spectrometry". Forensic Science International 99 (2): 93–105. January 1999. doi:10.1016/S0379-0738(98)00168-6. PMID 10077856.
- ↑ "Determination of psilocybin in Psilocybe semilanceata by capillary zone electrophoresis". Journal of Chromatography. B, Biomedical Sciences and Applications 694 (2): 375–381. July 1997. doi:10.1016/S0378-4347(97)00127-8. PMID 9252052.
- ↑ "A technique for the rapid isolation and identification of psilocin from psilocin/psilocybin-containing mushrooms". Journal of Forensic Sciences 30 (3): 931–941. July 1985. doi:10.1520/JFS11028J. PMID 4040953.
- ↑ "Analysis and isolation of indole alkaloids of fungi by high-performance liquid chromatography". Journal of Chromatography 593 (1–2): 201–208. 1992. doi:10.1016/0021-9673(92)80287-5.
- ↑ "Determination of psilocin in magic mushrooms and rat plasma by liquid chromatography with fluorimetry and electrospray ionization mass spectrometry". Analytica Chimica Acta 527 (2): 149–156. 2004. doi:10.1016/j.aca.2004.08.071. Bibcode: 2004AcAC..527..149S.
- ↑ 228.0 228.1 "Quantitation of psilocin in human plasma by high-performance liquid chromatography and electrochemical detection: comparison of liquid-liquid extraction with automated on-line solid-phase extraction". Journal of Chromatography. B, Biomedical Sciences and Applications 709 (2): 255–263. May 1998. doi:10.1016/S0378-4347(98)00067-X. PMID 9657222.
- ↑ "Analysis and characterization of psilocybin and psilocin using liquid chromatography-electrospray ionization mass spectrometry (LC-ESI-MS) with collision-induced-dissociation (CID) and source-induced dissociation (SID)". Microgram Journal 3 (3–4): 175–82. 2005. https://www.justice.gov/dea/programs/forensicsci/microgram/journal_v3_num34/journal_v3_num34_pg8.html.
- ↑ 230.0 230.1 "Detection of psilocin in body fluids". Forensic Science International 113 (1–3): 403–407. September 2000. doi:10.1016/S0379-0738(00)00213-9. PMID 10978655.
- ↑ "Determination of psilocin in rat urine by high-performance liquid chromatography with electrochemical detection". Journal of Chromatography 534: 287–290. December 1990. doi:10.1016/S0378-4347(00)82176-3. PMID 2094720.
- ↑ "The detection of psilocin in human urine". Journal of Forensic Sciences 46 (3): 627–630. May 2001. doi:10.1520/JFS15014J. PMID 11373000.
- ↑ "Optimized glucuronide hydrolysis for the detection of psilocin in human urine samples". Journal of Chromatography. B, Analytical Technologies in the Biomedical and Life Sciences 796 (2): 421–427. November 2003. doi:10.1016/j.jchromb.2003.08.030. PMID 14581081.
- ↑ "Development of a psilocin immunoassay for serum and blood samples". International Journal of Legal Medicine 118 (6): 326–331. December 2004. doi:10.1007/s00414-004-0469-9. PMID 15526212.
- ↑ "Use of high-temperature liquid chromatography with sub-2 μm particle C18 columns for the analysis of seized drugs". Journal of Liquid Chromatography & Related Technologies 32 (17–20): 2615–2626. 2009. doi:10.1080/10826070903245516. https://zenodo.org/record/1234491. Retrieved August 24, 2020.
- ↑ 236.0 236.1 236.2 236.3 236.4 236.5 Psilocybin Mushrooms of the World: An Identification Guide. Berkeley, California: Ten Speed Press. 1996. ISBN 978-0-89815-839-7.
- ↑ 237.0 237.1 "A worldwide geographical distribution of the neurotropic fungi, an analysis and discussion". Annali del Museo Civico di Rovereto: Sezione Archeologia, Storia, Scienze Naturali 14: 189–280. 2000. http://www.museocivico.rovereto.tn.it/UploadDocs/104_art09-Guzman%20&%20C.pdf. Retrieved October 19, 2021.
- ↑ 238.0 238.1 "Species diversity of the genus Psilocybe (Basidiomycotina, Agaricales, Strophariaceae) in the world mycobiota, with special attention to hallucinogenic properties". International Journal of Medicinal Mushrooms 7 (1–2): 305–331. 2005. doi:10.1615/intjmedmushr.v7.i12.280.
- ↑ "How Mushrooms Became Magic" (in en-US). The Atlantic. 24 August 2017. https://www.theatlantic.com/science/archive/2017/08/how-mushrooms-became-magic/537789/.
- ↑ 240.0 240.1 "Horizontal gene cluster transfer increased hallucinogenic mushroom diversity". Evolution Letters 2 (2): 88–101. April 2018. doi:10.1002/evl3.42. PMID 30283667.
- ↑ The Genus Psilocybe: A Systematic Revision of the Known Species Including the History, Distribution, and Chemistry of the Hallucinogenic Species. Beihefte Zur Nova Hedwigia. Heft 74. Vaduz, Liechtenstein: J. Cramer. 1983. pp. 361–362. ISBN 978-3-7682-5474-8.
- ↑ "Occurrence of psilocybin/psilocin in Pluteus salicinus (Plutaceae)". Mycologia 73 (4): 871–874. 1981. doi:10.2307/3759505. http://www.cybertruffle.org.uk/cyberliber/59350/0073/004/0781.htm. Retrieved August 29, 2011.
- ↑ "A worldwide geographical distribution of the neurotropic fungi, an analysis and discussion". Annali del Museo Civico di Rovereto 14: 207. 1998. http://www.magic-mushrooms.net/World_Wide_Distribution_of_Magic_Mushrooms.pdf. Retrieved September 17, 2017.
- ↑ "Analysis of psychotropic compounds in fungi of the genus Psilocybe by reversed-phase high performance liquid chromatography". Journal of Chromatography A 286: 229–235. 1984. doi:10.1016/S0021-9673(01)99190-3.
- ↑ "High-performance liquid chromatographic determination of some psychotropic indole derivatives". Journal of Chromatography 464 (2): 434–437. March 1989. doi:10.1016/s0021-9673(00)94264-x. PMID 2722990.
- ↑ 246.0 246.1 "Variation of psilocybin and psilocin levels with repeated flushes (harvests) of mature sporocarps of Psilocybe cubensis (Earle) Singer". Journal of Ethnopharmacology 5 (3): 287–291. May 1982. doi:10.1016/0378-8741(82)90014-9. PMID 7201054.
- ↑ "New aspects of the occurrence, chemistry and cultivation of European hallucinogenic mushrooms". Supplemento Agli Annali dei Musei Civici di Rovereto Sezione Archeologica, Storia e Scienze Naturali 8: 107–124. 1992.
- ↑ "Forensic analysis of hallucinogenic mushrooms and khat (Catha edulis Forsk) using cation-exchange liquid chromatography". Forensic Science International 195 (1–3): 160–164. February 2010. doi:10.1016/j.forsciint.2009.12.013. PMID 20047807.
- ↑ "Drug profiles: Hallucinogenic mushrooms". European Monitoring Centre for Drugs and Drug Addiction. September 19, 2011. http://www.emcdda.europa.eu/publications/drug-profiles/mushrooms.
- ↑ "The occurrence of psilocybin and psilocin in Finnish fungi". Journal of Natural Products 50 (4): 741–744. 1987. doi:10.1021/np50052a030. PMID 3430170. Bibcode: 1987JNAtP..50..741O.
- ↑ "Detecting psychoactive drugs in the developmental stages of mushrooms". Journal of Forensic Sciences 45 (3): 527–537. May 2000. doi:10.1520/JFS14725J. PMID 10855955. http://pdfs.semanticscholar.org/8dab/e1804d761d975ef16e28c2c994476d9122aa.pdf.
- ↑ "Biosynthesis of psilocybin. II. Incorporation of labelled tryptamine derivatives". Acta Chemica Scandinavica 22 (4): 1210–1218. 1968. doi:10.3891/acta.chem.scand.22-1210. PMID 5750023.
- ↑ 253.0 253.1 253.2 253.3 "Enzymatic Synthesis of Psilocybin". Angewandte Chemie 56 (40): 12352–12355. September 2017. doi:10.1002/anie.201705489. PMID 28763571. Bibcode: 2017ACIE...5612352F.
- ↑ "Modified E. coli pump out psilocybin". Chemical & Engineering News 97 (39): 11. 7 October 2019. doi:10.1021/cen-09739-scicon9. https://cen.acs.org/biological-chemistry/biotechnology/Modified-E-coli-pump-magic/97/i39. Retrieved December 3, 2019.
- ↑ "Metabolic engineering of Saccharomyces cerevisiae for the de novo production of psilocybin and related tryptamine derivatives". Metabolic Engineering 60: 25–36. July 2020. doi:10.1016/j.ymben.2019.12.007. PMID 32224264.
- ↑ "Reconstituting the complete biosynthesis of D-lysergic acid in yeast". Nature Communications 13 (1). February 2022. doi:10.1038/s41467-022-28386-6. PMID 35132076. Bibcode: 2022NatCo..13..712W.
- ↑ "The oldest representations of hallucinogenic mushrooms in the world (Sahara Desert, 9000-7000 BP)". Integration. Zeitschrift für geistbewegende Pflanzen und Kultur. 2/3: 69–65. 1992. https://www.academia.edu/79946409.
- ↑ "A prehistoric mural in Spain depicting neurotropic Psilocybe mushrooms?". Economic Botany 65 (2): 121–128. 2011. doi:10.1007/s12231-011-9152-5.
- ↑ 259.0 259.1 259.2 259.3 Chanterelle Dreams, Amanita Nightmares: The Love, Lore, and Mystique of Mushrooms. White River Junction, Vermont: Chelsea Green Publishing. 2010. ISBN 978-1603582148.
- ↑ 260.0 260.1 260.2 260.3 LSD, my problem child: reflections on sacred drugs, mysticism, and science. Santa Cruz, California: Multidisciplinary Association for Psychedelic Studies. 2009. ISBN 978-0979862229. https://archive.org/details/lsdmyproblemchil0000hofm_h2h0/mode/2up.
- ↑ "Religious use of hallucinogenic fungi: A comparison between Siberian and Mesoamerican Cultures". Karstenia 32 (2): 71–80. 1992. doi:10.29203/ka.1992.294.
- ↑ Soma: Divine Mushroom of Immortality. Harcourt Brace Jovanovick. 1968. p. 161. ISBN 978-0-88316-517-1.
- ↑ 263.0 263.1 Magic Mushrooms Around the World. Los Angeles, California: LIS Publications. 1997. ISBN 978-0-9653399-0-2.
- ↑ "Seeking the magic mushroom". Life: 101–120. May 13, 1957. ISSN 0024-3019. https://books.google.com/books?id=Jj8EAAAAMBAJ&pg=PA101. Retrieved February 27, 2016.
- ↑ "Notes préliminaires sur les agarics hallucinogènes du Mexique" (in fr). Revue de Mycologie 22 (1): 58–79. 1957.
- ↑ "A new behavior change program using psilocybin". Psychotherapy: Theory, Research & Practice 2 (2): 61–72. 1965. doi:10.1037/h0088612.
- ↑ "Human hallucinogen research: guidelines for safety". Journal of Psychopharmacology 22 (6): 603–620. August 2008. doi:10.1177/0269881108093587. PMID 18593734. PMC 3056407. http://csp.org/psilocybin/HopkinsHallucinogenSafety2008.pdf. Retrieved November 20, 2017.
- ↑ "Historical overview of psychoactive mushrooms". Inflammation and Regeneration 29 (1): 47–58. 2009. doi:10.2492/inflammregen.29.47.
- ↑ "The War on Drugs turns 50 today. It's time to make peace.". The Washington Post. https://www.washingtonpost.com/outlook/2021/06/17/war-drugs-turns-50-today-its-time-make-peace/.
- ↑ "Hallucinogens as medicine". Scientific American 303 (6): 77–79. 2010. doi:10.1038/scientificamerican1210-76. Bibcode: 2010SciAm.303f..76G. http://www.csp.org/psilocybin/SciAmHallucinogens201012.pdf. Retrieved July 25, 2011.
- ↑ 271.0 271.1 Pharmacotheon: Entheogenic Drugs, their Plant Sources and History. Kennewick, Washington: Natural Products Company. 1993. ISBN 978-0-9614234-3-8.
- ↑ Psilocybin: Magic Mushroom Grower's Guide (2nd ed.). San Francisco, California: Quick American Archives. 1991. ISBN 978-0-932551-06-1.
- ↑ "A laboratory method to obtain fruit from cased grain spawn of the cultivated mushoom, Agaricus bisporus". Mycologia 63 (1): 16–21. 1971. doi:10.2307/3757680. PMID 5102274. http://www.cybertruffle.org.uk/cyberliber/59350/0063/001/0016.htm. Retrieved September 7, 2011.
- ↑ "Psilocybin study hints at rebirth of hallucinogen research". Wired.com. July 1, 2008. https://www.wired.com/wiredscience/2008/07/psilocybin-stud/. Retrieved August 8, 2011.
- ↑ "A very memorable trip". sciencemag.org. July 1, 2008. https://www.science.org/content/article/very-memorable-trip.
- ↑ Mycophilia: Revelations from the Weird World of Mushrooms. New York, New York: Rodale. 2011. pp. 257–258. ISBN 978-1-60529-407-0. https://books.google.com/books?id=Br3PU2PJhywC&pg=PA258. Retrieved February 27, 2016.
- ↑ 277.0 277.1 Considering Alternatives to Psychedelic Drug Prohibition (Report). 27 June 2024. https://www.rand.org/pubs/research_reports/RRA2825-1.html.
- ↑ Annual report 2011: the state of the drugs problem in Europe (Report). Luxembourg: Publications Office of the European Union. November 2011. doi:10.2810/44330. ISBN 978-92-9168-470-0. http://www.emcdda.europa.eu/attachements.cfm/att_143743_EN_EMCDDA_AR2011_EN.pdf. Retrieved December 4, 2011.
- ↑ "Hallucinogenic mushrooms in Mexico: an overview". Economic Botany 62 (3): 404–412. 2008. doi:10.1007/s12231-008-9033-8. Bibcode: 2008EcBot..62..404G.
- ↑ "Caring for "Hassle-Free Highs" in Amsterdam.". Anthropology and Humanism 45 (2): 212–22. December 2020. doi:10.1111/anhu.12298.
- ↑ 281.0 281.1 (in Portuguese) Como mudar sua mente. Editora Intrinseca. 2018. ISBN 9788551004173.
- ↑ Psychedelic mysticism: Transforming consciousness, religious experiences, and voluntary peasants in postwar America.. Lexington Books. November 2015. ISBN 978-1-4985-0910-7.
- ↑ "DEA's Process for Religious Use of Psychedelics Needs More Consistent Standards, Government Watchdog Agency Says" (in en-US). 2024-07-15. https://doubleblindmag.com/deas-process-for-religious-use-of-psychedelics-needs-more-consistent-standards-government-watchdog-agency-says/.
- ↑ "Australian psychiatrists can now prescribe MDMA and psilocybin: who can access them and how do they work?" (in en-GB). The Guardian. 2023-06-30. ISSN 0261-3077. https://www.theguardian.com/australia-news/2023/jul/01/australian-psychiatrists-can-now-prescribe-mdma-and-psilocybin-who-can-access-them-and-how-do-they-work.
- ↑ Submission to the Western Australian Inquiry Into Alternative Approaches to Reducing Illicit Drug Use and Its Effects on the Community (Report). Australian Psychedelic Society Inc.. 30 January 2019. https://parliament.wa.gov.au/Parliament/commit.nsf/luInquiryPublicSubmissions/5F7C3C56F7ABD360482583A70009A934/$file/id.alt.063.190118.sub.Australian%20Psychedelic%20Society.pdf. Retrieved August 23, 2021.
- ↑ "Psychedelics not linked to mental health problems or suicidal behavior: a population study". Journal of Psychopharmacology 29 (3): 270–279. March 2015. doi:10.1177/0269881114568039. PMID 25744618.
- ↑ "The abuse potential of medical psilocybin according to the 8 factors of the Controlled Substances Act". Neuropharmacology 142: 143–166. November 2018. doi:10.1016/j.neuropharm.2018.05.012. PMID 29753748.
- ↑ "It's all you! Australian ayahuasca drinking, spiritual development, and immunitary individualism.". Critique of Anthropology 38 (3): 325–45. September 2018. doi:10.1177/0308275X18775818.
- ↑ "Laws in Motion to Bring 'Right to Try' Psychedelics at End-of-Life" (in en-US). 2024-05-21. https://hospicenews.com/2024/05/21/laws-in-motion-to-bring-right-to-try-psychedelics-at-end-of-life/.
- ↑ "Timothy Leary, Richard Alpert (Ram Dass) and the changing definition of psilocybin". The International Journal on Drug Policy 21 (3): 234–239. May 2010. doi:10.1016/j.drugpo.2009.08.004. PMID 19744846.
- ↑ "Potential Therapeutic Effects of Psilocybin: A Systematic Review". Cureus 14 (10). October 2022. doi:10.7759/cureus.30214. PMID 36381758.
- ↑ "Antidepressive, anxiolytic, and antiaddictive effects of ayahuasca, psilocybin and lysergic acid diethylamide (LSD): a systematic review of clinical trials published in the last 25 years". Therapeutic Advances in Psychopharmacology 6 (3): 193–213. June 2016. doi:10.1177/2045125316638008. PMID 27354908.
- ↑ "Rapid and sustained symptom reduction following psilocybin treatment for anxiety and depression in patients with life-threatening cancer: a randomized controlled trial". Journal of Psychopharmacology 30 (12): 1165–1180. December 2016. doi:10.1177/0269881116675512. PMID 27909164.
- ↑ "ACNP 62nd Annual Meeting: Poster Abstracts P251 – P500: P400. Psilocybin Therapy for Depression and Anxiety Associated With Parkinson's Disease: A Pilot Study". Neuropsychopharmacology 48 (Suppl 1): 211–354 (296–297). December 2023. doi:10.1038/s41386-023-01756-4. PMID 38040810.
- ↑ "ACNP 63rd Annual Meeting: Poster Abstracts P305-P608: P594. Psilocybin Therapy for Depression and Anxiety in Parkinson's Disease: an Open-Label Pilot Study". Neuropsychopharmacology 49 (Suppl 1): 236–417 (407–408). December 2024. doi:10.1038/s41386-024-02012-z. PMID 39643634.
- ↑ "COMPASS Pathways Receives FDA Breakthrough Therapy Designation for Psilocybin Therapy for Treatment-resistant Depression". Compass Pathways. https://compasspathways.com/compass-pathways-receives-fda-breakthrough-therapy-designation-for-psilocybin-therapy-for-treatment-resistant-depression/.
- ↑ "FDA tags psilocybin drug as clinical depression Breakthrough Therapy". Pharmaphorum. 2 December 2019. https://pharmaphorum.com/news/fda-tags-psilocybin-drug-as-clinical-depression-breakthrough-therapy/.
- ↑ "Lysergic Acid Diethylamide, Psilocybin and Dimethyltryptamine in Depression Treatment: A Systematic Review". Pharmaceuticals 14 (8): 793. August 2021. doi:10.3390/ph14080793. PMID 34451890.
- ↑ "Psychedelic renaissance: Revitalized potential therapies for psychiatric disorders". Drug Discov Today 28 (12). December 2023. doi:10.1016/j.drudis.2023.103818. PMID 37925136. https://drive.google.com/file/d/1mztRkW7vJu7KjpE1xKUyZoObWJ5QvEmt/view.
- ↑ "Psychedelic Commercialization: A Wide-Spanning Overview of the Emerging Psychedelic Industry". Psychedelic Medicine 1 (3): 150–165. 1 September 2023. doi:10.1089/psymed.2023.0013. ISSN 2831-4425. PMID 40046566. PMC 11661494. https://www.researchgate.net/publication/373171466.
- ↑ 301.0 301.1 301.2 301.3 301.4 301.5 301.6 "Current Understanding on Psilocybin for Major Depressive Disorder: A Review Focusing on Clinical Trials". Clin Psychopharmacol Neurosci 22 (2): 222–231. May 2024. doi:10.9758/cpn.23.1134. PMID 38627070.
- ↑ 302.0 302.1 "Single-Dose Psilocybin for a Treatment-Resistant Episode of Major Depression". New England Journal of Medicine 387 (18): 1637–1648. November 2022. doi:10.1056/NEJMoa2206443. PMID 36322843.
- ↑ "Single-Dose Psilocybin Treatment for Major Depressive Disorder: A Randomized Clinical Trial". JAMA 330 (9): 843–853. September 2023. doi:10.1001/jama.2023.14530. PMID 37651119.
- ↑ 304.0 304.1 "Results From a Long-Term Observational Follow-Up Study of a Single Dose of Psilocybin for a Treatment-Resistant Episode of Major Depressive Disorder". J Clin Psychiatry 86 (1). March 2025. doi:10.4088/JCP.24m15449. PMID 40047545.
- ↑ 305.0 305.1 "Trial of Psilocybin versus Escitalopram for Depression". New England Journal of Medicine 384 (15): 1402–1411. April 2021. doi:10.1056/NEJMoa2032994. PMID 33852780. https://www.nejm.org/doi/full/10.1056/NEJMoa2032994.
- ↑ 306.0 306.1 306.2 306.3 "Comparative oral monotherapy of psilocybin, lysergic acid diethylamide, 3,4-methylenedioxymethamphetamine, ayahuasca, and escitalopram for depressive symptoms: systematic review and Bayesian network meta-analysis". BMJ 386. August 2024. doi:10.1136/bmj-2023-078607. PMID 39168500.
- ↑ "Blinding and expectancy confounds in psychedelic randomized controlled trials". Expert Rev Clin Pharmacol 14 (9): 1133–1152. September 2021. doi:10.1080/17512433.2021.1933434. PMID 34038314.
- ↑ "A Critical Appraisal of Evidence on the Efficacy and Safety of Serotonergic Psychedelic Drugs as Emerging Antidepressants: Mind the Evidence Gap". J Clin Psychopharmacol 42 (6): 581–588. 2022. doi:10.1097/JCP.0000000000001608. PMID 36193898.
- ↑ "Risk of bias in randomized clinical trials on psychedelic medicine: A systematic review". J Psychopharmacol 37 (7): 649–659. July 2023. doi:10.1177/02698811231180276. PMID 37403379.
- ↑ "Expectancy Effects in Psychedelic Trials". Biol Psychiatry Cogn Neurosci Neuroimaging 9 (5): 512–521. May 2024. doi:10.1016/j.bpsc.2024.02.004. PMID 38387698.
- ↑ "Placebo Effect in the Treatment of Depression and Anxiety". Front Psychiatry 10. 2019. doi:10.3389/fpsyt.2019.00407. PMID 31249537.
- ↑ "False Beliefs in Academic Psychiatry: The Case of Antidepressant Drugs". Ethical Human Psychology and Psychiatry 20 (1): 6–16. July 2018. doi:10.1891/1559-4343.20.1.6. ISSN 1559-4343.
- ↑ "Culture, context, and ethics in the therapeutic use of hallucinogens: Psychedelics as active super-placebos?". Transcult Psychiatry 59 (5): 571–578. October 2022. doi:10.1177/13634615221131465. PMID 36263513.
- ↑ "Pharmacological, neural, and psychological mechanisms underlying psychedelics: A critical review". Neurosci Biobehav Rev 140. September 2022. doi:10.1016/j.neubiorev.2022.104793. PMID 35878791. "In addition, the strong prior expectations that many people have about psychedelics directly contribute to the psychedelic experience and as a consequence it has been suggested that psychedelics may act as a ‘super-placebo’ (Hartogsohn, 2016). Specifically, strong prior expectations (e.g., that a specific intervention will likely trigger a mystical experience) will increase the likelihood of having e.g., a mystical-type experience (Maij et al., 2019), and this placebo-effect is further boosted by the psychedelic-induced suggestibility.".
- ↑ 315.0 315.1 "Incremental efficacy systematic review and meta-analysis of psilocybin-for-depression RCTs". Psychopharmacology (Berl) 242 (10): 2139–2157. April 2025. doi:10.1007/s00213-025-06788-w. PMID 40266291.
- ↑ 316.0 316.1 "Efficacy and safety of eight enhanced therapies for treatment-resistant depression: A systematic review and network meta-analysis of RCTs". Psychiatry Res 339. September 2024. doi:10.1016/j.psychres.2024.116018. PMID 38924903.
- ↑ "Efficacy and Safety of Four Psychedelic-Assisted Therapies for Adults with Symptoms of Depression, Anxiety, and Posttraumatic Stress Disorder: A Systematic Review and Meta-Analysis". J Psychoactive Drugs 57 (1): 1–16. 2025. doi:10.1080/02791072.2023.2278586. PMID 37968944.
- ↑ "Psychedelic therapy for depressive symptoms: A systematic review and meta-analysis". J Affect Disord 322: 194–204. February 2023. doi:10.1016/j.jad.2022.09.168. PMID 36209780.
- ↑ 319.0 319.1 319.2 "Dose effect of psilocybin on primary and secondary depression: a preliminary systematic review and meta-analysis". J Affect Disord 296: 26–34. January 2022. doi:10.1016/j.jad.2021.09.041. PMID 34587546.
- ↑ 320.0 320.1 320.2 "Psilocybin-assisted therapy for depression: A systematic review and dose-response meta-analysis of human studies". Eur Neuropsychopharmacol 76: 61–76. November 2023. doi:10.1016/j.euroneuro.2023.07.011. PMID 37557019.
- ↑ 321.0 321.1 321.2 321.3 "Efficacy and safety of psilocybin in the treatment of Major Depressive Disorder (MDD): A dose-response network meta-analysis of randomized placebo-controlled clinical trials". Psychiatry Res 344. February 2025. doi:10.1016/j.psychres.2024.116337. PMID 39754904.
- ↑ "Randomized Controlled Trials of Psilocybin-Assisted Therapy in the Treatment of Major Depressive Disorder: Systematic Review and Meta-Analysis". Acta Psychiatr Scand 151 (5): 557–571. December 2024. doi:10.1111/acps.13778. PMID 39627679.
- ↑ "Assessing the effects of methodological differences on outcomes in the use of psychedelics in the treatment of anxiety and depressive disorders: A systematic review and meta-analysis". J Psychopharmacol 36 (1): 20–30. January 2022. doi:10.1177/02698811211044688. PMID 34519567.
- ↑ "Psilocybin-assisted psychotherapy for treatment resistant depression: A randomized clinical trial evaluating repeated doses of psilocybin". Med 5 (3): 190–200.e5. March 2024. doi:10.1016/j.medj.2024.01.005. PMID 38359838.
- ↑ 325.0 325.1 325.2 325.3 325.4 Hager, Sandy Brian (8 September 2025). "The shifting fortunes of corporate psychedelia". Finance and Society: 1–23. doi:10.1017/fas.2025.10014. ISSN 2059-5999. https://www.cambridge.org/core/services/aop-cambridge-core/content/view/A2E9484FA459A5AC584E6F70DE24D5A3/S2059599925100149a.pdf/div-class-title-the-shifting-fortunes-of-corporate-psychedelia-div.pdf. "The second development is the announcement of the first readout from Compass Pathways' phase 3 trial of COMP360 in late June 2025. The announcement had been described as a 'make or break moment' for the leading company in the field (Hardman, 2025). Compass Pathways (2025) declared the results 'highly statistically significant and clinically meaningful'.".
- ↑ 326.0 326.1 326.2 326.3 326.4 326.5 326.6 "Compass points to psychedelic therapy's phase 3 depression win, but investors unimpressed". 23 June 2025. https://www.fiercebiotech.com/biotech/compass-points-psychedelic-therapys-phase-3-win-investors-unimpressed.
- ↑ 327.0 327.1 327.2 "Updated: Compass claims Phase 3 win in depression for psilocybin drug, yet stock plummets". 23 June 2025. https://endpoints.news/compass-reports-phase-3-depression-data-for-psilocybin-drug/.
- ↑ 328.0 328.1 328.2 "Compass Pathways succeeds in phase III depression study; stock slides". 28 June 2025. https://www.bioworld.com/articles/721464-compass-pathways-succeeds-in-phase-iii-depression-study-stock-slides.
- ↑ Moncrieff, Joanna (16 January 2025). "Alternative Approaches: The Good, the Bad and the Worrying: Psychedelics for Depression". Chemically Imbalanced: The Making and Unmaking of the Serotonin Myth. Flint. ISBN 978-1-80399-680-6. https://books.google.com/books?id=e0MkEQAAQBAJ. Retrieved 16 October 2025.
External links
- Psilocybin - Isomer Design
- 4-HO-DMT (Psilocybin) - TiHKAL - Erowid
- 4-HO-DMT (Psilocybin) - TiHKAL - Isomer Design
- Psilocybin Investigator's Brochure, Version 4.1 (2021) - Usona Institute
Template:Navboxes #invoke:Navbox
 |


