Chemistry:ETFELA
From HandWiki
Short description: Chemical compound
 | |
| Clinical data | |
|---|---|
| Other names | LA-CH2CF3 |
| Identifiers | |
| |
| Chemical and physical data | |
| Formula | C20H22F3N3O |
| Molar mass | 377.411 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
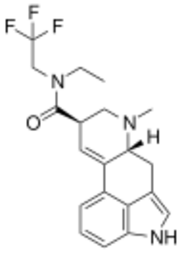
ETFELA (N-ethyl-N-(2,2,2-trifluoroethyl)lysergamide) is an analog of lysergic acid diethylamide (LSD) first synthesised by Jason C. Parrish as part of the research team led by David E. Nichols. In studies in vitro, it was found to be slightly more potent than LSD itself.[1][2]
See also
References
- ↑ Nichols, David E. (2012). "Structure-activity relationships of serotonin 5-HT2A agonists". Wiley Interdisciplinary Reviews: Membrane Transport and Signaling 1 (5): 559–579. doi:10.1002/wmts.42.
- ↑ "Chemistry and Structure-Activity Relationships of Psychedelics". Current Topics in Behavioral Neurosciences 36: 1–43. 2017. doi:10.1007/7854_2017_475. ISBN 978-3-662-55878-2. PMID 28401524.
| Lysergic acid derivatives |
|
|---|---|
| Psychedelic lysergamides | |
| clavines | |
| Other ergolines | |
| Natural sources |
Morning glory: Argyreia nervosa (Hawaiian Baby Woodrose), Ipomoea spp.(Morning Glory, Tlitliltzin, Badoh Negro), Rivea corymbosa (Coaxihuitl, Ololiúqui) |
 | 0.00      (0 votes) (0 votes) |