Chemistry:Arsenic tribromide
| |
| Names | |
|---|---|
| Preferred IUPAC name
Arsenic tribromide | |
| Systematic IUPAC name
Tribromoarsane | |
| Other names
Arsenic(III) bromide
Arsenous bromide, Arsenicum Bromatum, Tribromoarsine | |
| Identifiers | |
3D model (JSmol)
|
|
| ChemSpider | |
| EC Number |
|
PubChem CID
|
|
| RTECS number |
|
| UNII | |
| |
| |
| Properties | |
| AsBr 3 | |
| Molar mass | 314.634 g/mol |
| Appearance | white to pale yellow crystalline solid |
| Density | 3.54 g/cm3 |
| Melting point | 31.1 °C (88.0 °F; 304.2 K) |
| Boiling point | 221 °C (430 °F; 494 K) |
| soluble, partial hydrolysis indicated by fumes | |
| -106.0·10−6 cm3/mol | |
Refractive index (nD)
|
2.3 |
| Hazards | |
| NIOSH (US health exposure limits): | |
PEL (Permissible)
|
[1910.1018] TWA 0.010 mg/m3[1] |
REL (Recommended)
|
Ca C 0.002 mg/m3 [15-minute][1] |
IDLH (Immediate danger)
|
Ca [5 mg/m3 (as As)][1] |
| Related compounds | |
Related compounds
|
Phosphorus tribromide arsenic trichloride |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
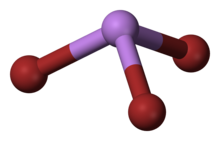
Arsenic tribromide is an inorganic compound with the formula AsBr
3, it is a bromide of arsenic. Arsenic is a chemical element that has the symbol As and atomic number 33. This pyramidal molecule is the only known binary arsenic bromide. AsBr
3 is noteworthy for its very high refractive index of approximately 2.3. It also has a very high diamagnetic susceptibility.[2] It is a poisonous metalloid that has many allotropic forms: yellow (molecular non-metallic) and several black and gray forms (metalloids), orthorhombic prisms, colorless rhombic crystals are a few that are seen. Bromine is a halogen element with the symbol Br and atomic number 35. Diatomic bromine does not occur naturally, but bromine salts can be found in crustal rock.[2]
Preparation
Arsenic tribromide can be prepared by the direct bromination of arsenic powder. Alternatively, arsenic(III) oxide can be used as the precursor in the presence of elemental sulfur:
2 As
2O
3 + 3 S + 6 Br
2 → 5 ASBr
3 + 3 SO
2
Arsenic tribromide is a highly water soluble crystalline arsenic source for uses compatible with bromides and lower (acidic) pH. Most metal bromide compounds are water soluble for uses in water treatment, chemical analysis and in ultra high purity for certain crystal growth applications. Arsenic bromide is generally immediately available in most volumes.[3]
It is soluble in hydrocarbons; carbon tetrachloride; very soluble in ether, benzene, chlorinated hydrocarbons, carbon disulfide, oils, and fats.
Bromides of arsenic
AsBr
5 is not known, although the corresponding phosphorus compound PBr
5 is well characterized. AsBr
3 is the parent for a series of hypervalent anionic bromoarsenates including [AS
2Br
8]2−, [AS
2Br
9]3−, and [AS
3Br
12]3−.[4]
Organoarsenic bromides (CH
3)
2AsBr and (CH
3)AsBr
2 are formed efficiently by the copper-catalyzed reaction of methyl bromide with hot arsenic metal. This synthesis is similar to the direct process used for the synthesis of methyl chlorosilanes.
Safety
Arsenic tribromide is highly toxic and may be fatal if inhaled, swallowed or absorbed through skin. Fire may produce irritating, corrosive and/or toxic gases. Based on sufficient evidence from human data. It is a carcinogen and a teratogen.
An increased lung cancer mortality was observed in multiple human populations exposed primarily through inhalation. Increased mortality from multiple internal organ cancers (liver, kidney, lung, and bladder) and an increased incidence of skin cancer were observed in populations consuming drinking water high in inorganic arsenic.[5]
Breathing arsenic bromide can irritate the nose and throat and can cause an ulcer or hole in the inner nose. High exposure can cause poor appetite, nausea, vomiting, diarrhea, muscle cramps, convulsions and death. Arsenic tribromide may lower the red blood cell count, damage the liver and kidneys. High or repeated exposure may damage the nerves causing weakness, "pins and needles," and poor coordination in arms and legs.[6]
References
- ↑ 1.0 1.1 1.2 NIOSH Pocket Guide to Chemical Hazards. "#0038". National Institute for Occupational Safety and Health (NIOSH). https://www.cdc.gov/niosh/npg/npgd0038.html.
- ↑ 2.0 2.1 CRC handbook of Chemistry and Physics, CRC Press
- ↑ "Arsenic Tribromide" in Handbook of Preparative Inorganic Chemistry, 2nd ed. Edited by G. Brauer, Academic Press, 1963, NY. Vol. 1. p. 597.
- ↑ Holleman, A. F.; Wiberg, E. "Inorganic Chemistry" Academic Press: San Diego, 2001. ISBN:0-12-352651-5.
- ↑ PubChem. "Hazardous Substances Data Bank (HSDB) : 425" (in en). https://pubchem.ncbi.nlm.nih.gov/source/hsdb/425.
- ↑ "New Jersey Department of Health and Senior Services". July 2000. https://www.nj.gov/health/eoh/rtkweb/documents/fs/0154.pdf.
 |

