Chemistry:Dibromine trioxide
From HandWiki
| |
| Names | |
|---|---|
| IUPAC name
Dibromine trioxide
| |
| Other names
Bromine trioxide
Bromine bromate | |
| Identifiers | |
3D model (JSmol)
|
|
| ChemSpider | |
PubChem CID
|
|
| |
| |
| Properties | |
| Br2O3 | |
| Molar mass | 207.806 g/mol |
| Appearance | orange needles |
| Melting point | decomposes around −40°C[1] |
| Structure[2] | |
| monoclinic | |
| P21/c | |
a = 1186.6 pm, b = 762.9 pm, c = 869.3 pm α = 90°, β = 106.4°, γ = 90°
| |
| Related compounds | |
Other anions
|
Bromine dioxide Bromine trifluoride Bromine pentafluoride |
Other cations
|
Oxygen difluoride Dichlorine monoxide Chlorine dioxide Iodine dioxide |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
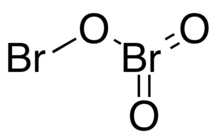
Dibromine trioxide is the chemical compound composed of bromine and oxygen with the formula Br2O3. It is an orange solid that is stable below −40 °C. It has the structure Br−O−BrO2 (bromine bromate).[3] It was discovered at 1993.[2] The bond angle of Br−O−Br is 111.7°, the bond angle of O−Br=O is 103.1°, and the bond angle of O=Br=O is 107.6°. The Br−OBrO2 bond length is 1.845Å, the O−BrO2 bond length is 1.855Å, and the Br=O bond length is 1.612Å.[4]
Reactions
Dibromine trioxide can be prepared by reacting a solution of bromine in dichloromethane with ozone at low temperatures.[3][5] It disproportionates in alkali solutions to Br− and BrO−3.[5]
References
- ↑ Perry, Dale L.; Phillips, Sidney L. (1995), Handbook of Inorganic Compounds, CRC Press, pp. 255, ISBN 0-8493-8671-3, https://books.google.com/books?id=0fT4wfhF1AsC&q=%22Mercury(I)+bromide%22&pg=PA255, retrieved 2015-08-25
- ↑ 2.0 2.1 Kuschel, Raimund; Seppelt, Konrad (1993). "Brombromat Br2O3". Angewandte Chemie (Wiley) 105 (11): 1734–1735. doi:10.1002/ange.19931051141. ISSN 0044-8249.
- ↑ 3.0 3.1 Henderson, K. M. Mackay; R. A. Mackay; W. (2002). Introduction to modern inorganic chemistry (6th ed.). Cheltenham: Nelson Thornes. ISBN 9780748764204.
- ↑ Jansen, Martin; Kraft, Thorsten (1997). "The Structural Chemistry of Binary Halogen Oxides in the Solid State". Chemische Berichte (Wiley) 130 (3): 307–316. doi:10.1002/cber.19971300302. ISSN 0009-2940.
- ↑ 5.0 5.1 Wiberg, Egon (2001). Wiberg, Nils. ed. Inorganic chemistry (1st ed.). San Diego, Calif.: Academic Press. pp. 464. ISBN 9780123526519.
 |

