Chemistry:Bromine dioxide
| |
| |
| Names | |
|---|---|
| IUPAC name
Bromine dioxide
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChemSpider | |
PubChem CID
|
|
| |
| |
| Properties | |
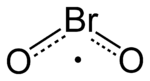
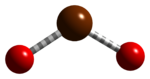
| BrO2 | |
| Molar mass | 111.903 g/mol[1] |
| Appearance | unstable yellow crystals |
| Melting point | decomposes around 0°C |
| Related compounds | |
Other anions
|
Bromine monoxide Bromine trifluoride Bromine pentafluoride |
Other cations
|
Oxygen difluoride Dichlorine monoxide Chlorine dioxide Iodine dioxide |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Bromine dioxide is the chemical compound composed of bromine and oxygen with the formula BrO2. It forms unstable yellow[2] to yellow-orange[1] crystals. It was first isolated by R. Schwarz and M. Schmeißer in 1937 and is hypothesized to be important in the atmospheric reaction of bromine with ozone.[3] It is similar to chlorine dioxide, the dioxide of its halogen neighbor one period higher on the periodic table.[citation needed]
Reactions
Bromine dioxide is formed when an electric current is passed through a mixture of bromine and oxygen gases at low temperature and pressure.[4]
Bromine dioxide can also be formed by the treatment of bromine gas with ozone in trichlorofluoromethane at −50 °C.[1]
When mixed with a base, bromine dioxide gives the bromide and bromate anions:[4]
References
- ↑ 1.0 1.1 1.2 Perry, Dale L.; Phillips, Sidney L. (1995), Handbook of Inorganic Compounds, CRC Press, pp. 74, ISBN 0-8493-8671-3, https://books.google.com/books?id=0fT4wfhF1AsC&q=%22Bromine+dioxide%22&pg=PA74, retrieved 17 March 2009
- ↑ 2.0 2.1 Lide, David R. (1998), Handbook of Chemistry and Physics (87 ed.), Boca Raton, Florida: CRC Press, pp. 447, ISBN 0-8493-0594-2
- ↑ Müller, Holger S. P.; Miller, Charles E.; Cohen, Edward A. (1997). "The rotational spectrum and molecular properties of bromine dioxide, OBrO". The Journal of Chemical Physics 107 (20): 8292. doi:10.1063/1.475030. ISSN 0021-9606. Bibcode: 1997JChPh.107.8292M.
- ↑ 4.0 4.1 Arora, M.G. (1997), P-Block Elements, New Delhi: Anmol Publications, pp. 256, ISBN 978-81-7488-563-0, https://books.google.com/books?id=QR3TCaKaykEC&q=%22Bromine+dioxide%22&pg=PA256, retrieved 17 March 2009
 |
