Chemistry:Arsenic trichloride
| |
| Names | |
|---|---|
| Other names
Arsenic(III) chloride, Arsenous trichloride, Arsine trichloride, Butter of arsenic, de Valagin's solution, Trichloroarsine
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChemSpider | |
| EC Number |
|
PubChem CID
|
|
| RTECS number |
|
| UNII | |
| UN number | 1560 |
| |
| |
| Properties | |
| AsCl3 | |
| Molar mass | 181.28 g/mol |
| Appearance | colourless oily liquid |
| Density | 2.163 g/cm3, liquid |
| Melting point | −13 °C (9 °F; 260 K) |
| Boiling point | 181 °C (358 °F; 454 K) |
| Hydrolyzes | |
| Solubility | soluble in alcohol, ether, HCl, HBr, chloroform, CCl4[1] |
| -79.9·10−6 cm3/mol | |
Refractive index (nD)
|
1.6006 |
| Viscosity | 9.77 x 10−6 Pa s |
| Hazards | |
| Main hazards | Very toxic, carcinogen, corrosive, decomposes on contact with water releasing HCl |
| GHS pictograms |    
|
| GHS Signal word | Danger |
| H301, H310, H314, H331, H350, H410 | |
| P201, P202, P260, P261, P262, P264, P270, P271, P273, P280, P281, P301+310, P301+330+331, P302+350, P303+361+353, P304+340, P305+351+338, P308+313, P310, P311, P321, P322, P330, P361, P363 | |
| NFPA 704 (fire diamond) | |
| Lethal dose or concentration (LD, LC): | |
LD50 (median dose)
|
48 mg/kg |
LCLo (lowest published)
|
100 mg/m3 (cat, 1 hr) 200 mg/m3 (cat, 20 min) 338 ppm (rat, 10 min)[3] |
| NIOSH (US health exposure limits): | |
PEL (Permissible)
|
[1910.1018] TWA 0.010 mg/m3[2] |
REL (Recommended)
|
Ca C 0.002 mg/m3 [15-minute][2] |
IDLH (Immediate danger)
|
Ca [5 mg/m3 (as As)][2] |
| Related compounds | |
Other anions
|
Arsenic trioxide, Arsenic trifluoride |
Other cations
|
Antimony trichloride |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Arsenic trichloride is an inorganic compound with the formula AsCl3, also known as arsenous chloride or butter of arsenic. This poisonous oil is colourless, although impure samples may appear yellow. It is an intermediate in the manufacture of organoarsenic compounds.[4]
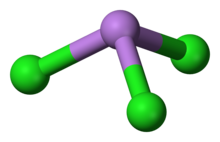
Structure
AsCl3 is a pyramidal molecule with C3v symmetry. The As-Cl bond is 2.161 Å and the angle Cl-As-Cl is 98° 25'±30.[5][6] AsCl3 has four normal modes of vibration: ν1(A1) 416, ν2(A1) 192, ν3 393, and ν4(E) 152 cm−1.[7]
Synthesis
This colourless liquid is prepared by treatment of arsenic(III) oxide with hydrogen chloride followed by distillation:
- As2O3 + 6 HCl → 2 AsCl3 + 3 H2O
It can also be prepared by chlorination of arsenic at 80–85 °C, but this method requires elemental arsenic.[4]
- 2 As + 3 Cl2 → 2 AsCl3
Arsenic trichloride can be prepared by the reaction of arsenic oxide and sulfur monochloride. This method requires simple apparatus and proceeds efficiently:[8]
- 2 As2O3 + 6 S2Cl2 → 4 AsCl3 + 3 SO2 + 9 S
A convenient laboratory method is refluxing arsenic(III) oxide with thionyl chloride:[9]
- 2 As2O3 + 3 SOCl2 → 2 AsCl3 + 3 SO2
Arsenic trichloride can also be prepared by the reaction of hydrochloric acid and arsenic(III) sulfide.
- As2S3 + 6 HCl → 2 AsCl3 + 3 H2S
Reactions
Hydrolysis gives arsenous acid and hydrochloric acid:
- AsCl3 + 3 H2O → As(OH)3 + 3 HCl
Although AsCl3 is less moisture sensitive than PCl3, it still fumes in moist air.[10]
AsCl3 undergoes redistribution upon treatment with As2O3 to give the inorganic polymer AsOCl. With chloride sources, AsCl3, forms salts containing the anion [AsCl4]−. Reaction with potassium bromide and potassium iodide give arsenic tribromide and arsenic triiodide, respectively.
AsCl3 is useful in organoarsenic chemistry, for example triphenylarsine is derived from AsCl3:[11]
- AsCl3 + 6 Na + C6H5Cl → As(C6H5)3 + 6 NaCl
The chemical weapons called Lewisites are prepared by the addition of arsenic trichloride to acetylene:
- AsCl
3 + C
2H
2 → ClCH=CHAsCl
2
Safety
Inorganic arsenic compounds are highly toxic,[4] and AsCl3 especially so because of its volatility and solubility (in water).
It is classified as an extremely hazardous substance in the United States as defined in Section 302 of the U.S. Emergency Planning and Community Right-to-Know Act (42 U.S.C. 11002), and is subject to strict reporting requirements by facilities which produce, store, or use it in significant quantities.[12]
References
- ↑ John Rumble (June 18, 2018) (in English). CRC Handbook of Chemistry and Physics (99th ed.). CRC Press. pp. 4–41. ISBN 978-1138561632.
- ↑ 2.0 2.1 2.2 NIOSH Pocket Guide to Chemical Hazards. "#0038". National Institute for Occupational Safety and Health (NIOSH). https://www.cdc.gov/niosh/npg/npgd0038.html.
- ↑ "Arsenic (inorganic compounds, as As)". Immediately Dangerous to Life and Health Concentrations (IDLH). National Institute for Occupational Safety and Health (NIOSH). https://www.cdc.gov/niosh/idlh/7440382.html.
- ↑ 4.0 4.1 4.2 Sabina C. Grund, Kunibert Hanusch, Hans Uwe Wolf "Arsenic and Arsenic Compounds" in Ullmann's Encyclopedia of Industrial Chemistry, VCH-Wiley, 2008, Weinheim.doi:10.1002/14356007.a03_113.pub2
- ↑ P. Kisliuk; C. H. Townes. "The Microwave Spectra and Molecular Structure of Phosphorus and Arsenic Trichloride". J. Chem. Phys. 1950, 18.
- ↑ Jean Galy; Renee Enjalbertl Pierre Lecante; Andrzej Burian "AsCl3: From the crystalline to the liquid state. XRD (176< T (K) < 250) and WAXS (295K) studies" Inorg. Chem 2002, volume 41, pp. 693–698.doi:10.1021/ic0102788
- ↑ Klapoetke, Thomas M. "The vibrational spectrum of arsenic trichloride" Main Group Metal Chemistry 1997, volume 20, pp. 81–83.
- ↑ R. C. Smith, "Manufacture of Arsenic trichloride" The Journal of Industrial and Engineering Chemistry 1919, volume 11, pp. 109–110. doi:10.1021/ie50110a009
- ↑ Pandey, S. K.; Steiner, A.; Roesky, H. W. (1997). "Arsenic(III) chloride". Inorganic Synthesis 31: 148-150. doi:10.1002/9780470132623.ch24.
- ↑ Holleman, A. F.; Wiberg, E. Inorganic Chemistry Academic Press: San Diego, 2001. ISBN:0-12-352651-5.
- ↑ Shriner, R. L.; Wolf, C. N. (1963). "Tetraphenylarsonium Chloride Hydrochloride". Organic Syntheses. http://www.orgsyn.org/demo.aspx?prep=cv4p0910.; Collective Volume, 4, pp. 910. Describes the preparation of As(C6H5)3.
- ↑ 40 C.F.R.: Appendix A to Part 355—The List of Extremely Hazardous Substances and Their Threshold Planning Quantities (Report) (July 1, 2008 ed.). Government Printing Office. http://edocket.access.gpo.gov/cfr_2008/julqtr/pdf/40cfr355AppA.pdf. Retrieved October 29, 2011.
 |


