Chemistry:Bromocyclopropane
From HandWiki
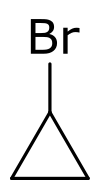
| |
| Names | |
|---|---|
| Preferred IUPAC name
Bromocyclopropane | |
| Other names
Cyclopropyl bromide, cyclopropylbromide
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChemSpider | |
| EC Number |
|
PubChem CID
|
|
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| C3H5Br | |
| Molar mass | 120.977 g·mol−1 |
| Appearance | liquid |
| Density | 1.515 g/cm3 |
| Boiling point | 68–70 °C (154–158 °F; 341–343 K) |
| Insoluble | |
| Hazards | |
| GHS pictograms |  
|
| GHS Signal word | Danger |
| H225, H315, H319, H335 | |
| P210, P233, P240, P241, P242, P243, P261, P264, P264+265Script error: No such module "Preview warning".Category:GHS errors, P271, P280, P302+352, P303+361+353, P304+340, P305+351+338, P319Script error: No such module "Preview warning".Category:GHS errors, P321, P332+317Script error: No such module "Preview warning".Category:GHS errors, P337+317Script error: No such module "Preview warning".Category:GHS errors, P362+364Script error: No such module "Preview warning".Category:GHS errors, P370+378, P403+233, P403+235, P405, P501 | |
| Related compounds | |
Related compounds
|
Chlorocyclopropane Fluorocyclopropane Iodocyclopropane |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Tracking categories (test):
Bromocyclopropane is a organobromine compound with the chemical formula C
3H
5Br.[2] It is a member of haloalkane family.
Synthesis
The compound can be obtained by treating silver cyclopropanecarboxylate with bromine:[3]
- C
3H
5CO
2Ag + Br
2 → C
3H
5Br + AgBr + CO
2
Chemical properties
Reaction with magnesium, using diethyl ether as solvent, only gives about 25–30% of the cyclopropylmagnesium bromide Grignard reagent, with nearly as much cyclopropane formed by alternate reactions on the metal surface.[4]
The compound isomerizes on heating to produce 1-bromopropene and 3-bromopropene.[5]
Physical properties
The compound is a flammable liquid that causes skin irritation and serious eye irritation. It is soluble in chloroform and methanol. Insoluble in water.[6]
See also
- Bromoalkanes
- Bromocyclohexane
References
- ↑ "Bromocyclopropane" (in en). https://pubchem.ncbi.nlm.nih.gov/compound/78037#section=Safety-and-Hazards.
- ↑ "Bromocyclopropane". Sigma Aldrich. https://www.sigmaaldrich.com/RU/en/product/aldrich/c117307.
- ↑ Roberts, John D.; Chambers, Vaughan C. (July 1951). "Small-Ring Compounds. VI. Cyclopropanol, Cyclopropyl Bromide and Cyclopropylamine" (in en). Journal of the American Chemical Society 73 (7): 3176–3179. doi:10.1021/ja01151a053. ISSN 0002-7863.
- ↑ Walborsky, H. M.; Zimmermann, Christoph (June 1992). "The surface nature of Grignard reagent formation. Cyclopropylmagnesium bromide" (in en). Journal of the American Chemical Society 114 (13): 4996–5000. doi:10.1021/ja00039a007. ISSN 0002-7863.
- ↑ Grant, R. C. S.; Swinbourne, E. S. (1 January 1966). "The thermal isomerization of chlorocyclopropane and of bromocyclopropane" (in en). Chemical Communications (17): 620b–621. doi:10.1039/C1966000620B. ISSN 0009-241X.
- ↑ "Bromocyclopropane, 99%". Alfa Aesar. https://www.alfa.com/ru/catalog/A14996/.
 |

