Chemistry:Bulevirtide
 | |
| Clinical data | |
|---|---|
| Trade names | Hepcludex |
| Other names | MyrB, Myrcludex-B[1] |
| License data | |
| Routes of administration | Subcutaneous |
| ATC code | |
| Legal status | |
| Legal status | |
| Identifiers | |
| CAS Number | |
| DrugBank | |
| UNII | |
| KEGG | |
| ChEMBL | |
| Chemical and physical data | |
| Formula | C248H355N65O72 |
| Molar mass | 5398.951 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
Bulevirtide, sold under the brand name Hepcludex, is an antiviral medication for the treatment of chronic hepatitis D (in the presence of hepatitis B).[3]
The most common side effects include raised levels of bile salts in the blood and reactions at the site of injection.[3]
Bulevirtide works by attaching to and blocking a receptor (target) through which the hepatitis delta and hepatitis B viruses enter liver cells.[3] By blocking the entry of the virus into the cells, it limits the ability of HDV to replicate and its effects in the body, reducing symptoms of the disease.[3]
Bulevirtide was approved for medical use in the European Union in July 2020.[3]
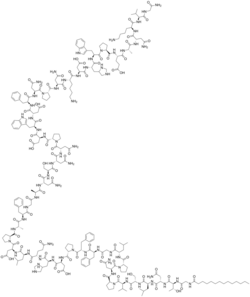
Structural formula
Bulevirtide is a 47-amino acid peptide with the following sequence:[5]
CH3(CH2)12CO-Gly-Thr-Asn-Leu-Ser-Val-Pro-Asn-Pro-Leu-Gly-Phe-Phe-Pro-Asp-His-Gln-Leu-Asp-Pro-Ala-Phe-Gly-Ala-Asn-Ser-Asn-Asn-Pro-Asp-Trp-Asp-Phe-Asn-Pro-Asn-Lys-Asp-His-Trp-Pro-Glu-Ala-Asn-Lys-Val-Gly-NH2 (C13H27CO-GTNLSVPNPLGFFPDHQLDPAFGANSNNPDWDFNPNKDHWPEANKVG-NH2)
Medical uses
Bulevirtide is indicated for the treatment of chronic hepatitis delta virus (HDV) infection in plasma (or serum) HDV-RNA positive adult patients with compensated liver disease.[3][6]
Pharmacology
Mechanism of action
Bulevirtide binds and inactivates the sodium/bile acid cotransporter, blocking both HBV and HDV viruses from entering hepatocytes.[7]
The hepatitis B virus uses its surface lipopeptide pre-S1 for docking to mature liver cells via their sodium/bile acid cotransporter (NTCP) and subsequently entering the cells. Myrcludex B is a synthetic N-acylated pre-S1[8][9] that can also dock to NTCP, blocking the virus's entry mechanism.[10]
The drug is also effective against hepatitis D because the hepatitis D virus uses the same entry receptor as HBV and is only effective in the presence of a hepatitis B virus infection.[10]
Pre-clinical data in mice suggests that pharmacological inhibition of NTCP-mediated bile salt uptake may also be effective to lower hepatic bile salt accumulation in cholestatic conditions. This reduces hepatocellular damage.[11] An increased ratio of phospholipid to bile salts seen in bile upon NTCP inhibition may further contribute to the protective effect as bile salts are less toxic in presence of phospholipids.[12]
References
- ↑ "Beyond Pegylated Interferon-Alpha: New Treatments for Hepatitis Delta". AIDS Reviews 21 (3): 126–134. 2019. doi:10.24875/AIDSRev.19000080. PMID 31532397.
- ↑ "Hepcludex 2 mg powder for solution for injection - Summary of Product Characteristics (SmPC)". 30 March 2022. https://www.medicines.org.uk/emc/product/13482.
- ↑ 3.0 3.1 3.2 3.3 3.4 3.5 3.6 "Hepcludex EPAR". 26 May 2020. https://www.ema.europa.eu/en/medicines/human/EPAR/hepcludex. Text was copied from this source which is © European Medicines Agency. Reproduction is authorized provided the source is acknowledged.
- ↑ "Hepcludex Product information". https://ec.europa.eu/health/documents/community-register/html/h1446.htm.
- ↑ "Intact plasma quantification of the large therapeutic lipopeptide bulevirtide". Analytical and Bioanalytical Chemistry 413 (22): 5645–5654. September 2021. doi:10.1007/s00216-021-03384-7. PMID 34018034.
- ↑ "Summary of opinion: Hepcludex". European Medicines Agency. 28 May 2020. https://www.ema.europa.eu/en/documents/smop-initial/chmp-summary-positive-opinion-hepcludex_en.pdf.
- ↑ "Hepcludex". 29 May 2020. https://www.ema.europa.eu/en/medicines/human/summaries-opinion/hepcludex.
- ↑ "The entry inhibitor Myrcludex-B efficiently blocks intrahepatic virus spreading in humanized mice previously infected with hepatitis B virus". Journal of Hepatology 58 (5): 861–867. May 2013. doi:10.1016/j.jhep.2012.12.008. PMID 23246506.
- ↑ "Management of hepatitis delta: Need for novel therapeutic options". World Journal of Gastroenterology 21 (32): 9461–9465. August 2015. doi:10.3748/wjg.v21.i32.9461. PMID 26327754.
- ↑ 10.0 10.1 "Neue Wirkstoffe – Myrcludex B" (in German). Österreichische Apothekerzeitung (19/2015): 12. 14 September 2015.
- ↑ Na+ -taurocholate cotransporting polypeptide inhibition has hepatoprotective effects in cholestasis in mice. Slijepcevic D, Roscam Abbing RLP, Fuchs CD, Haazen LCM, Beuers U, Trauner M, Oude Elferink RPJ, van de Graaf SFJ. Hepatology. 2018 Sep;68(3):1057-1069. doi: 10.1002/hep.29888
- ↑ "Blocking Sodium-Taurocholate Cotransporting Polypeptide Stimulates Biliary Cholesterol and Phospholipid Secretion in Mice". Hepatology 71 (1): 247–258. January 2020. doi:10.1002/hep.30792. PMID 31136002.
External links
- "Bulevirtide". Drug Information Portal. U.S. National Library of Medicine. https://druginfo.nlm.nih.gov/drugportal/name/bulevirtide.
 |

