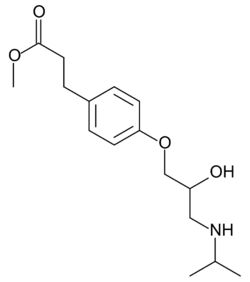
Chemistry:Esmolol
 | |
| Clinical data | |
|---|---|
| Trade names | Brevibloc |
| AHFS/Drugs.com | Monograph |
| Pregnancy category |
|
| Routes of administration | Intravenous |
| ATC code | |
| Legal status | |
| Legal status | |
| Pharmacokinetic data | |
| Protein binding | 60% |
| Metabolism | Red blood cell (erythrocytic) |
| Elimination half-life | 9 minutes |
| Excretion | Kidney |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| IUPHAR/BPS | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEBI | |
| ChEMBL | |
| Chemical and physical data | |
| Formula | C16H25NO4 |
| Molar mass | 295.379 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| | |
Esmolol, sold under the brand name Brevibloc, is a cardio selective beta1 receptor blocker with rapid onset,[3] a very short duration of action, and no significant intrinsic sympathomimetic or membrane stabilising activity at therapeutic dosages.
It is a class II antiarrhythmic.[4] Esmolol decreases the force and rate of heart contractions by blocking beta-adrenergic receptors of the sympathetic nervous system, which are found in the heart and other organs of the body. Esmolol prevents the action of two naturally occurring substances: epinephrine and norepinephrine.[5]
It was patented in 1980 and approved for medical use in 1987.[6]
Medical uses
To terminate supraventricular tachycardia,
Episodic atrial fibrillation or flutter,
Arrhythmia during anaesthesia,
To reduce HR and BP during and after cardiac surgery, and
In early treatment of myocardial infarction.
Esmolol is also used in blunting the hemodynamic response to laryngoscopy and intubation.[7]
Pharmacology
Pharmacodynamics
Esmolol is a beta blocker, or an antagonist of the β-adrenergic receptors.[8] It is selective for the β1-adrenergic receptor and has no intrinsic sympathomimetic activity.[8]
Pharmacokinetics
Esmolol is considered a soft drug,[9] one that is rapidly metabolized to an inactive form. Esmolol is rapidly metabolized by hydrolysis of the ester linkage, chiefly by the esterases in the cytosol of red blood cells and not by plasma cholinesterases or red cell membrane acetylcholinesterase. Total body clearance in man was found to be about 20 L/kg/hr, which is greater than cardiac output; thus the metabolism of esmolol is not limited by the rate of blood flow to metabolizing tissues such as the liver or affected by hepatic or renal blood flow. Esmolol's short duration of action is based on the ester-methyl side chain which allows for quick hydrolysis. Esmolol's structure is reflected in its name, es-molol as in ester-methyl. Plasma cholinesterases and red cell membrane acetylcholinesterase do not have any action. This metabolism results in the formation of a free acid and methanol. The amount of methanol produced is similar to endogenous methanol production. Esmolol has a rapid distribution half-life of about two minutes and an elimination half-life of about nine minutes.[citation needed]
Esmolol is classified as a beta blocker with low lipophilicity and hence lower potential for crossing the blood–brain barrier.[8] This in turn may result in fewer effects in the central nervous system as well as a lower risk of neuropsychiatric side effects.[8]
References
- ↑ "Brevibloc esmolol hydrochloride 2.5 g powder for injection for infusion vial (310943)". 26 May 2022. https://www.tga.gov.au/resources/artg/310943.
- ↑ "Esmolol Juno (Juno Pharmaceuticals Pty Ltd)". 13 January 2023. https://www.tga.gov.au/resources/prescription-medicines-registrations/esmolol-juno-juno-pharmaceuticals-pty-ltd.
- ↑ "Esmolol inhibits Na+ current in rat ventricular myocytes". Methods and Findings in Experimental and Clinical Pharmacology 28 (10): 697–702. December 2006. doi:10.1358/mf.2006.28.10.1037498. PMID 17235414. http://journals.prous.com/journals/servlet/xmlxsl/pk_journals.xml_summaryn_pr?p_JournalId=6&p_RefId=1037498. Retrieved 2008-07-27.
- ↑ "Recent antiarrhythmic drugs". The American Journal of Cardiology 64 (20): 65J–69J. December 1989. doi:10.1016/0002-9149(89)91203-4. PMID 2688391.
- ↑ "Antiadrenergic Drugs and Drugs for Glaucoma". Essentials of Medical Pharmacology (7th ed.). p. 149.
- ↑ (in en) Analogue-based Drug Discovery. John Wiley & Sons. 2006. p. 462. ISBN 978-3-527-60749-5. https://books.google.com/books?id=FjKfqkaKkAAC&pg=PA462.
- ↑ "Comparison of Esmolol and Dexmedetomidine for Suppression of Hemodynamic Response to Laryngoscopy and Endotracheal Intubation in Adult Patients Undergoing Elective General Surgery: A Prospective, Randomized Controlled Double-blinded Study". Anesthesia: Essays and Researches 12 (1): 262–266. 2018. doi:10.4103/aer.AER_226_17. PMID 29628593.
- ↑ 8.0 8.1 8.2 8.3 "Neuropsychiatric Consequences of Lipophilic Beta-Blockers". Medicina (Kaunas) 57 (2): 155. February 2021. doi:10.3390/medicina57020155. PMID 33572109.
- ↑ "Soft drug design: General principles and recent applications". Medicinal Research Reviews 20 (1): 58–101. 2000. doi:10.1002/(SICI)1098-1128(200001)20:1<58::AID-MED3>3.0.CO;2-X. PMID 10608921.
 |
