Chemistry:Nadolol
 | |
| Clinical data | |
|---|---|
| Trade names | Corgard, others |
| AHFS/Drugs.com | Monograph |
| MedlinePlus | a682666 |
| License data | |
| Pregnancy category |
|
| Routes of administration | By mouth |
| Drug class | Beta blocker |
| ATC code | |
| Legal status | |
| Legal status | |
| Pharmacokinetic data | |
| Protein binding | 30% |
| Metabolism | Not metabolised |
| Elimination half-life | 14-24 hours |
| Excretion | Renal and fecal (unchanged) |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| IUPHAR/BPS | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEMBL | |
| Chemical and physical data | |
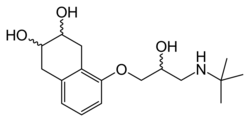
| Formula | C17H27NO4 |
| Molar mass | 309.406 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| (verify) | |
Nadolol, sold under the brand name Corgard among others, is a medication used to treat high blood pressure, heart pain, atrial fibrillation, and some inherited arrhythmic syndromes.[1] It has also been used to prevent migraine headaches and complications of cirrhosis.[2][3] It is taken orally.[2]
Common side effects include dizziness, feeling tired, a slow heart rate, and Raynaud syndrome.[1] Serious side effects may include heart failure and bronchospasm.[1] Its use in pregnancy and breastfeeding is of unclear safety.[4] It is a non-selective beta blocker and works by blocking β1-adrenergic receptors in the heart and β2-adrenergic receptors in blood vessels.[1]
Nadolol was patented in 1970 and came into medical use in 1978.[5] It is available as a generic medication.[1] In 2020, it was the 340th most commonly prescribed medication in the United States, with more than 700 thousand prescriptions.[6]
Medical uses
Nadolol is used to treat hypertension and for long-term treatment of angina pectoris and is approved by the FDA for these purposes.[7]
It is regularly used off-label[7] for control of heart rate in people with atrial fibrillation,[8] prevention of migraine headaches;[9] prevention of bleeding veins in people with portal hypertension caused by cirrhosis;[3] and to treat people with high levels of thyroid hormone.[10]
Nadolol is the preferred beta-blocker in the management of patients with LQTS for prevention of ventricular arrhythmia. It is more efficacious than selective beta blockers or propranolol in the prevention of breakthrough cardiac events.[11] Similarly, it is the preferred type of beta blocker for treatment of patients with CPVT, as it has been shown to be more efficacious than selective beta blockers, like atenolol or bisoprolol.[12]
Nadolol has the advantage of once daily dosing and thus improved patient compliance. For patients with decreased kidney function, nadolol may be dosed less often.[13] It has also been found to be useful (off-label) for several neurological disorders such as the prevention of migraine attacks,[14] attention deficit/hyperactivity disorder(ADHD)[15] and its use has been explored as a treatment for essential tremor[16] and Parkinson's disease[17] but neither is well established.[18][19][20]
Side effects
The most common side effects include dizziness and fatigue.[17]
Contraindications
Nadolol and other beta blockers should be used with cautions in people with heart failure and its use should not be abruptly stopped. It is contraindicated for people with asthma, a slow heart rate and certain severe heart problems.[21]
Pharmacology
Pharmacodynamics

Nadolol is a non-selective beta blocker; that is, it non-selectively blocks both beta-1 and beta-2 receptors. It has a preference for beta-1 receptors, which are predominantly located in the heart, thereby inhibiting the effects of catecholamines and causing a decrease in heart rate and blood pressure. Its inhibition of beta-2 receptors, which are mainly located in the bronchial smooth muscle of the airways, leads to airway constriction similar to that seen in asthma. Inhibition of beta-1 receptors in the juxtaglomerular apparatus of the kidney inhibits the renin–angiotensin system, causing a decrease in vasoconstriction and a decrease in water retention. Nadolol's inhibition of beta-1 receptors in the heart and kidney leads to its effects on lowering blood pressure.
The drug impairs AV node conduction and decreases sinus rate.
Nadolol may also increase plasma triglycerides and decrease HDL-cholesterol levels. [citation needed]
Pharmacokinetics
Nadolol is classified as a beta blocker with low lipophilicity and hence lower potential for crossing the blood–brain barrier.[22] This in turn may result in fewer effects in the central nervous system as well as fewer neuropsychiatric side effects.[22]
Chemistry
Nadolol is a mixture of stereoisomers. It is polar and hydrophilic, with low lipid solubility.[23]
References
- ↑ 1.0 1.1 1.2 1.3 1.4 "Nadolol Monograph for Professionals". American Society of Health-System Pharmacists. https://www.drugs.com/monograph/nadolol.html.
- ↑ 2.0 2.1 British national formulary : BNF 76 (76 ed.). Pharmaceutical Press. 2018. pp. 148. ISBN 9780857113382.
- ↑ 3.0 3.1 "Beta-blockers in liver cirrhosis". Annals of Gastroenterology 27 (1): 20–26. 2014. PMID 24714633.
- ↑ "Nadolol Pregnancy and Breastfeeding Warnings". https://www.drugs.com/pregnancy/nadolol.html.
- ↑ Analogue-based Drug Discovery. John Wiley & Sons. 2006. p. 460. ISBN 9783527607495. https://books.google.com/books?id=FjKfqkaKkAAC&pg=PA460.
- ↑ "Nadolol - Drug Usage Statistics". https://clincalc.com/DrugStats/Drugs/Nadolol.
- ↑ 7.0 7.1 Nadolol entry in AccessMedicine. McGraw-Hill Global Education Holdings, LLC. Accessed 8 November 2014
- ↑ "2014 AHA/ACC/HRS guideline for the management of patients with atrial fibrillation: a report of the American College of Cardiology/American Heart Association Task Force on practice guidelines and the Heart Rhythm Society". Circulation 130 (23): e199–e267. December 2014. doi:10.1161/CIR.0000000000000041. PMID 24682347.
- ↑ "Evidence-based guideline update: pharmacologic treatment for episodic migraine prevention in adults: report of the Quality Standards Subcommittee of the American Academy of Neurology and the American Headache Society". Neurology 78 (17): 1337–1345. April 2012. doi:10.1212/WNL.0b013e3182535d20. PMID 22529202.
- ↑ "Hyperthyroidism and other causes of thyrotoxicosis: management guidelines of the American Thyroid Association and American Association of Clinical Endocrinologists". Thyroid 21 (6): 593–646. June 2011. doi:10.1089/thy.2010.0417. PMID 21510801.
- ↑ "Interplay Between Genetic Substrate, QTc Duration, and Arrhythmia Risk in Patients With Long QT Syndrome". Journal of the American College of Cardiology 71 (15): 1663–1671. April 2018. doi:10.1016/j.jacc.2018.01.078. PMID 29650123.
- ↑ "Outcomes of Patients With Catecholaminergic Polymorphic Ventricular Tachycardia Treated With β-Blockers". JAMA Cardiology 7 (5): 504–512. May 2022. doi:10.1001/jamacardio.2022.0219. PMID 35353122.
- ↑ "Corgard (nadolol) dosing, indications, interactions, adverse effects, and more". http://reference.medscape.com/drug/corgard-nadolol-342361.
- ↑ "Nadolol - a beta-blocker - Corgard. High blood pressure drugs". http://patient.info/medicine/nadolol-a-beta-blocker-corgard.
- ↑ Attention-deficit Hyperactivity Disorder: A Clinical Workbook. Guilford Press. 27 May 2017. ISBN 9781593852276. https://books.google.com/books?id=EkyTTvjNRZAC&q=Nadolol+for+ADHD&pg=PA669. Retrieved 27 May 2017.
- ↑ "Evidence-based guideline update: treatment of essential tremor: report of the Quality Standards subcommittee of the American Academy of Neurology". Neurology 77 (19): 1752–1755. November 2011. doi:10.1212/WNL.0b013e318236f0fd. PMID 22013182.
- ↑ 17.0 17.1 U.S. National Library of Medicine Nadolol entry in Medline Plus
- ↑ "Peripheral beta-adrenergic blockade treatment of parkinsonian tremor". Annals of Neurology 16 (4): 505–508. October 1984. doi:10.1002/ana.410160412. PMID 6149724.
- ↑ "Nadolol: MedlinePlus Drug Information". https://www.nlm.nih.gov/medlineplus/druginfo/meds/a682666.html.
- ↑ "Nadolol Dosage Guide with Precautions - Drugs.com". https://www.drugs.com/dosage/nadolol.html.
- ↑ "Corgard Label". http://www.accessdata.fda.gov/drugsatfda_docs/label/2008/018063s060lbl.pdf.
- ↑ 22.0 22.1 "Neuropsychiatric Consequences of Lipophilic Beta-Blockers". Medicina (Kaunas) 57 (2): 155. February 2021. doi:10.3390/medicina57020155. PMID 33572109.
- ↑ "Optimized separation of beta-blockers with multiple chiral centers using capillary electrochromatography-mass spectrometry". Journal of Chromatography. B, Analytical Technologies in the Biomedical and Life Sciences 875 (1): 304–316. November 2008. doi:10.1016/j.jchromb.2008.06.028. PMID 18619928.
External links
- "Nadolol". Drug Information Portal. U.S. National Library of Medicine. https://druginfo.nlm.nih.gov/drugportal/name/nadolol.
 |
