Chemistry:Tocainide
Tocainide (Tonocard) is a class Ib antiarrhythmic agent. It is no longer sold in the United States.
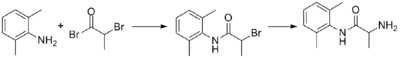
Synthesis
Pharmacokinetics
Tocainide is a lidocaine derivative, that undergoes very less first pass metabolism. It occurs as two enantiomers. The R isomer is three times more potent than the S isomer.[5] Tocainide's oral bioavailability is almost 100%.[6] Plasma half-life generally lasts for 11.5-15.5 hours (13.5 ± 2 hours[7]). In the blood, tocainide is 10-20% protein bound.[8][6] The volume of distribution is 2.8-3.2 L/kg.[8] 31-45% is excreted unchanged in the urine.[8] The more active R-isomer is cleared faster in anephric patients (without kidneys) or those with severe kidney dysfunction. The main metabolite is tocainide carbamoyl ester glucuronlde.[9]
Drug interactions
Rifampicin increases conversion of tocainide into its main metabolite, tocainide carbamoyl ester glucuronlde,[9] by inducing the glucuronosyl transferase enzyme that catalyzes glucuronidation of tocainide to produce that metabolite. Rifampicin also increases elimination rate and decreases oral clearance of tocainide.[10] Tocainide decreases plasma clearance of theophylline.[11]
Pharmacokinetics
Oral bioavailability is high (near complete), with low protein binding (≈10–20%) and renal excretion of a substantial unchanged fraction; metabolism proceeds largely via glucuronidation. Reported plasma half-life values are roughly in the 9–20 hour range, with known stereoselective differences between enantiomers.[12][13]
History and status
Tocainamide (tocainide) emerged in the 1970s as an orally effective alternative to lidocaine for ventricular arrhythmias, with early uncontrolled and controlled studies in refractory patients.[14][15] Accumulating reports of bone-marrow toxicity and other serious adverse events led to declining use and eventual withdrawal from some markets; in the United States, tocainide products were removed in 2003.[16][17]
See also
- Class I antiarrhythmic
- Mexiletine
- Lidocaine
References
- ↑ Boyes RN, Byrnes EW, "Antiarrhythmisch Wirksame Verbindung, Verfahren zu Deren Herstellung und Deren Verwendung", DE patent 2235745, issued 1972, assigned to Astra Pharmaceutical Products Inc.
- ↑ "Primary Amino Acylanilides Methods of Making the Same and Use as Antiarrhythmic Drugs" GB patent 1461602, issued 1974, assigned to Astra Pharmaceutical Products Inc.
- ↑ Boyes RN, Duce BR, Smith EM, Byrnes EW, "Primaeraminoacylanilide, Verfahren zu Deren Herstellung und Sie Enthaltende Arzneimittel", DE patent 2400540, issued 1974, assigned to Astra Pharmaceutical Products Inc.
- ↑ "New antiarrhythmic agents. 1. Primary alpha-amino anilides". Journal of Medicinal Chemistry 22 (10): 1171–1176. October 1979. doi:10.1021/jm00196a005. PMID 513064.
- ↑ "Stereoselective interaction of tocainide and its chiral analogs with the sodium channels in human myoballs". Pflugers Archiv 418 (3): 234–237. April 1991. doi:10.1007/BF00370521. PMID 1649990.
- ↑ 6.0 6.1 "Tocainide: a new oral antiarrhythmic agent". Annals of Internal Medicine 103 (3): 387–391. September 1985. doi:10.7326/0003-4819-103-3-387. PMID 3927807.
- ↑ "Clinical efficacy and pharmacokinetics of a new orally effective antiarrhythmic, tocainide". Circulation 54 (6): 885–889. December 1976. doi:10.1161/01.CIR.54.6.885. PMID 791536.
- ↑ 8.0 8.1 8.2 "Kidney Disease Program (KDP)". University of Louisville. https://kdpnet.kdp.louisville.edu/drugbook/adult/?leaf=4314.
- ↑ 9.0 9.1 Kwok DW (1987). Studies on the metabolism of tocainide in humans (Thesis). doi:10.14288/1.0096967. hdl:2429/26428.[page needed]
- ↑ "Influence of rifampin on tocainide pharmacokinetics in humans". Clinical Pharmacy 8 (3): 200–205. March 1989. PMID 2495879.
- ↑ "The effect of tocainide on theophylline metabolism". British Journal of Clinical Pharmacology 35 (4): 437–440. April 1993. doi:10.1111/j.1365-2125.1993.tb04163.x. PMID 8485025.
- ↑ "Drug therapy. Flecainide". The New England Journal of Medicine 315 (1): 36–41. July 1986. doi:10.1056/NEJM198607033150106. PMID 3520324.
- ↑ "A Focus on the Synthesis and Pharmacokinetics of Tocainide and its Analogues". Current Medicinal Chemistry 25 (42): 5822–5834. 6 February 2019. doi:10.2174/0929867325666180327104320. PMID 29589531.
- ↑ "Tocainide for refractory symptomatic ventricular arrhythmias". The American Journal of Cardiology 49 (5): 1279–1286. April 1982. doi:10.1016/0002-9149(82)90056-X. PMID 6801955.
- ↑ "Short- and long-term therapy with tocainide for malignant ventricular tachyarrhythmias". Circulation 73 (1): 143–149. January 1986. doi:10.1161/01.cir.73.1.143. PMID 3079677.
- ↑ "Tocainide". Drugs and Lactation Database (LactMed®). National Institute of Child Health and Human Development. 2006. https://www.ncbi.nlm.nih.gov/books/NBK501117/.
- ↑ "The Treatment of Trigeminal Neuralgia". Nerves and Nerve Injuries. 2015. pp. 81–97. doi:10.1016/B978-0-12-802653-3.00055-5. ISBN 978-0-12-802653-3.
Further reading
- Applied Pharmacokinetics & Pharmacodynamics: Principles of Therapeutic Drug Monitoring. Lippincott Williams & Wilkins. 2006. ISBN 978-0-7817-4431-7. OCLC 59148565.
External links
 |
