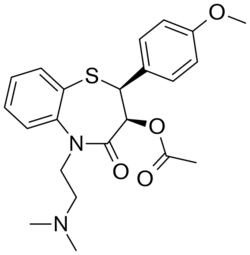
Chemistry:Diltiazem
 | |
 | |
| Clinical data | |
|---|---|
| Pronunciation | /dɪlˈtaɪəzɛm/ |
| Trade names | Cardizem, Dilacor XR, Cartia XT, others |
| AHFS/Drugs.com | Monograph |
| MedlinePlus | a684027 |
| License data | |
| Pregnancy category |
|
| Routes of administration | By mouth, intravenous |
| Drug class | Nondihydropyridine calcium channel blocker |
| ATC code | |
| Legal status | |
| Legal status | |
| Pharmacokinetic data | |
| Bioavailability | 40% |
| Metabolism | Liver |
| Elimination half-life | 3–4.5 hours |
| Excretion | Kidney Bile duct |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| IUPHAR/BPS | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEBI | |
| ChEMBL | |
| PDB ligand | |
| Chemical and physical data | |
| Formula | C22H26N2O4S |
| Molar mass | 414.52 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| (verify) | |
Diltiazem, sold under the brand name Cardizem among others, is a nondihydropyridine calcium channel blocker medication used to treat high blood pressure, angina, and certain heart arrhythmias.[8] It may also be used in hyperthyroidism if beta blockers cannot be used.[8] It is taken by mouth or injection into a vein.[8] When given by injection, effects typically begin within a few minutes and last a few hours.[8]
Common side effects include swelling, dizziness, headaches, and low blood pressure.[8] Other severe side effects include an overly slow heart beat, heart failure, liver problems, and allergic reactions.[8] Use is not recommended during pregnancy.[8] It is unclear if use when breastfeeding is safe.[1]
Diltiazem works by relaxing the smooth muscle in the walls of arteries, resulting in them opening and allowing blood to flow more easily.[8] Additionally, it acts on the heart to prolong the period until it can beat again.[9] It does this by blocking the entry of calcium into the cells of the heart and blood vessels.[10] It is a class IV antiarrhythmic.[11]
Diltiazem was approved for medical use in the United States in 1982.[8] It is available as a generic medication.[8] In 2020, it was the 76th most commonly prescribed medication in the United States, with more than 9 million prescriptions.[12][13] An extended release formulation is also available.[8][14]
Medical uses
Diltiazem is indicated for:
- Stable angina (exercise-induced) – diltiazem increases coronary blood flow and decreases myocardial oxygen consumption, secondary to decreased peripheral resistance, heart rate, and contractility.[15][16]
- Variant angina – it is effective owing to its direct effects on coronary dilation.
- Unstable angina (preinfarction, crescendo) – diltiazem may be particularly effective if the underlying mechanism is vasospasm.
- Myocardial bridge
For supraventricular tachycardias (PSVT), diltiazem appears to be as effective as verapamil in treating re-entrant supraventricular tachycardia.[17]
Atrial fibrillation[18] or atrial flutter is another indication. The initial bolus should be 0.25 mg/kg, intravenous (IV).
Because of its vasodilatory effects, diltiazem is useful for treating hypertension. Calcium channel blockers are well tolerated, and especially effective in treating low-renin hypertension.[19]
It is also used as topical application for anal fissures because it promotes healing due to its vasodilatory property.[20]
Contraindications and precautions
- In congestive heart failure, patients with reduced ventricular function may not be able to counteract the negative inotropic and chronotropic effects of diltiazem, the result being an even higher compromise of function.
- With SA node or AV conduction disturbances, the use of diltiazem should be avoided in patients with SA or AV nodal abnormalities, because of its negative chronotropic and dromotropic effects.
- Low blood pressure patients, with systolic blood pressures below 90 mm Hg, should not be treated with diltiazem.
- Diltiazem may paradoxically increase ventricular rate in patients with Wolff-Parkinson-White syndrome because of accessory conduction pathways.
Diltiazem is relatively contraindicated in the presence of sick sinus syndrome, atrioventricular node conduction disturbances, bradycardia, impaired left ventricle function, peripheral artery occlusive disease, and chronic obstructive pulmonary disease.
Side effects
A reflex sympathetic response, caused by the peripheral dilation of vessels and the resulting drop in blood pressure, works to counteract the negative inotropic, chronotropic and dromotropic effects of diltiazem. Undesirable effects include hypotension, bradycardia, dizziness, flushing, fatigue, headaches and edema.[21] Rare side effects are congestive heart failure, myocardial infarction, and hepatotoxicity.[22]
Diltiazem is one of the most common drugs that cause drug-induced lupus, along with hydralazine, procainamide, isoniazid, minocycline.[23]
Drug interactions
Because of its inhibition of hepatic cytochromes CYP3A4, CYP2C9 and CYP2D6, there are a number of drug interactions.[24] Some of the more important interactions are listed below.
Beta-blockers
Intravenous diltiazem should be used with caution with beta-blockers because, while the combination is most potent at reducing heart rate, there are rare instances of dysrhythmia and AV node block.[25]
Quinidine
Quinidine should not be used concurrently with calcium channel blockers because of reduced clearance of both drugs and potential pharmacodynamic effects at the SA and AV nodes.[26]
Fentanyl
Concurrent use of fentanyl with diltiazem, or any other CYP3A4 inhibitors, as these medications decrease the breakdown of fentanyl and thus increase its effects.[27]
Mechanism of action

Diltiazem, also known as (2S,3S)-3-acetoxy-5-[2-(dimethylamino)ethyl]-2,3-dihydro-2-(4-methoxyphenyl)-1,5-benzothiazepin-4(5H)-one hydrochlorid has a vasodilating activity attributed to the (2S,3S)-isomer.[28] Diltiazem is a potent vasodilator, increasing blood flow and variably decreasing the heart rate via strong depression of A-V node conduction. It binds to the alpha-1 subunit of L-type calcium channels in a fashion somewhat similar to verapamil, another nondihydropyridine (non-DHP) calcium channel blocker.[29] Chemically, it is based upon a 1,4-thiazepine ring, making it a benzothiazepine-type calcium channel blocker.
It is a potent and mild vasodilator of coronary and peripheral vessels, respectively,[30] which reduces peripheral resistance and afterload, though not as potent as the dihydropyridine (DHP) calcium channel blockers. This results in minimal reflexive sympathetic changes.[citation needed]
Diltiazem has negative inotropic, chronotropic, and dromotropic effects. This means diltiazem causes a decrease in heart muscle contractility – how strong the beat is, lowering of heart rate – due to slowing of the sinoatrial node, and a slowing of conduction through the atrioventricular node – increasing the time needed for each beat. Each of these effects results in reduced oxygen consumption by the heart, reducing angina, typically unstable angina, symptoms. These effects also reduce blood pressure by causing less blood to be pumped out.
Research
Diltiazem is prescribed off-label by doctors in the US for prophylaxis of cluster headaches. Some research on diltiazem and other calcium channel antagonists in the treatment and prophylaxis of migraine is ongoing.[15][31][32][33][34][35][36][needs update]
Recent research[when?] has shown diltiazem may reduce cocaine cravings in drug-addicted rats.[37] This is believed to be due to the effects of calcium blockers on dopaminergic and glutamatergic signaling in the brain.[38] Diltiazem also enhances the analgesic effect of morphine in animal tests, without increasing respiratory depression,[39] and reduces the development of tolerance.[40]
Diltiazem is also being used in the treatment of anal fissures. It can be taken orally or applied topically with increased effectiveness.[41] When applied topically, it is made into a cream form using either vaseline or Phlojel. Phlojel absorbs the diltiazem into the problem area better than the vaseline base. It has good short-term success rates.[42][43]
References
- ↑ 1.0 1.1 "Diltiazem Use During Pregnancy". 4 May 2020. https://www.drugs.com/pregnancy/diltiazem.html.
- ↑ "Dilcardia SR 120 mg Prolonged-release hard capsules – Summary of Product Characteristics (SmPC)". 22 March 2018. https://www.medicines.org.uk/emc/product/2584/smpc.
- ↑ "Angitil SR/XL Capsules – Summary of Product Characteristics (SmPC)". 7 May 2019. https://www.medicines.org.uk/emc/product/7509/smpc.
- ↑ "Cardizem- diltiazem hydrochloride tablet, coated". 2 June 2020. https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=f3e7ecef-f360-4987-a4f5-933214130ab2.
- ↑ "Cardizem CD- diltiazem hydrochloride capsule, coated, extended release". 30 April 2020. https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=1042fa13-e6af-46b9-8008-6c941f0978b1.
- ↑ "Cardizem LA- diltiazem hydrochloride tablet, extended release". 2 May 2019. https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=3e180dc7-c871-4efc-8755-cae882976c8d.
- ↑ "Active substance(s): diltiazem". European Medicines Agency. 11 January 2018. https://www.ema.europa.eu/en/documents/psusa/diltiazem-list-nationally-authorised-medicinal-products-psusa/00001084/201705_en.pdf.
- ↑ 8.00 8.01 8.02 8.03 8.04 8.05 8.06 8.07 8.08 8.09 8.10 "Diltiazem Hydrochloride Monograph for Professionals". AHFS. https://www.drugs.com/monograph/diltiazem-hydrochloride.html.
- ↑ Cardiovascular Pharmacotherapeutics. Cardiotext Publishing. 2011. pp. 251–52. ISBN 978-1935395621. https://books.google.com/books?id=Yi9ZAgAAQBAJ&pg=PA252. Retrieved 28 December 2018.
- ↑ 2010 Nurse's Drug Handbook. Jones & Bartlett Learning. 2010. p. 320. ISBN 978-0763779009. https://archive.org/details/2010nursesdrugha0000unse.
- ↑ Gardner's Commercially Important Chemicals: Synonyms, Trade Names, and Properties. John Wiley & Sons. 2005. p. 223. ISBN 978-0471736615. https://books.google.com/books?id=oWdc2qcb3QsC&pg=PA223. Retrieved 28 December 2018.
- ↑ "The Top 300 of 2020". https://clincalc.com/DrugStats/Top300Drugs.aspx.
- ↑ "Diltiazem – Drug Usage Statistics". https://clincalc.com/DrugStats/Drugs/Diltiazem.
- ↑ "Diltiazem hydrochloride – diltiazem hydrochloride extended-release tablets tablet, extended release". 1 April 2019. https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=73b3607a-99d0-44d8-92ef-0074684c9d7d.
- ↑ 15.0 15.1 "Calcium antagonists". Progress in Cardiovascular Diseases 47 (1): 34–57. 2004. doi:10.1016/j.pcad.2004.04.006. PMID 15517514.
- ↑ "Long-acting diltiazem HCl for the chronotherapeutic treatment of hypertension and chronic stable angina pectoris". Expert Opinion on Pharmacotherapy 6 (5): 765–776. May 2005. doi:10.1517/14656566.6.5.765. PMID 15934903.
- ↑ "Diltiazem to treat sinus tachycardia in critically ill patients: a four-year experience". Critical Care Medicine 29 (10): 1874–1879. October 2001. doi:10.1097/00003246-200110000-00004. PMID 11588443.
- ↑ "Acute ventricular rate control in atrial fibrillation: IV combination of diltiazem and digoxin vs. IV diltiazem alone". Chest 119 (2): 502–506. February 2001. doi:10.1378/chest.119.2.502. PMID 11171729.
- ↑ "The role of existing and newer calcium channel blockers in the treatment of hypertension". Journal of Clinical Hypertension 6 (11): 621–629; quiz 630–631. November 2004. doi:10.1111/j.1524-6175.2004.03683.x. PMID 15538095.
- ↑ "The role of topical diltiazem in the treatment of chronic anal fissures that have failed glyceryl trinitrate therapy". Colorectal Disease 4 (6): 430–435. October 2002. doi:10.1046/j.1463-1318.2002.00376.x. PMID 12790914.
- ↑ "A one-year evaluation of calcium channel blocker overdoses: toxicity and treatment". Annals of Emergency Medicine 22 (2): 196–200. February 1993. doi:10.1016/S0196-0644(05)80202-1. PMID 8427431.
- ↑ "Diltiazem". StatPearls. Treasure Island (FL): StatPearls Publishing. 2019.
- ↑ "article-24529". Drug-Induced Lupus Erythematosus. Treasure Island (FL): StatPearls Publishing. 2019. http://www.ncbi.nlm.nih.gov/books/NBK441889/. Retrieved 20 November 2019.
- ↑ "General framework for the quantitative prediction of CYP3A4-mediated oral drug interactions based on the AUC increase by coadministration of standard drugs". Clinical Pharmacokinetics 46 (8): 681–696. 2007. doi:10.2165/00003088-200746080-00005. PMID 17655375.
- ↑ "Cardiovascular adverse drug reaction associated with combined beta-adrenergic and calcium entry-blocking agents". Journal of Cardiovascular Pharmacology 35 (4): 556–559. April 2000. doi:10.1097/00005344-200004000-00007. PMID 10774785.
- ↑ "Effects of atrio-ventricular conduction of calcium-antagonistic coronary vasodilators, local anaesthetics and quinidine injected into the posterior and the anterior septal artery of the atrio-ventricular node preparation of the dog". Naunyn-Schmiedeberg's Archives of Pharmacology 294 (2): 169–177. August 1976. doi:10.1007/bf00507850. PMID 1012337.
- ↑ "Drug interactions: Interactions between fentanyl and drugs that inhibit CYP3A4". https://www.pharmaceutical-journal.com/learning/learning-article/drug-interactions-interactions-between-fentanyl-and-drugs-that-inhibit-cyp3a4/10039015.article.
- ↑ "Diltiazem Synthesis". Encyclopedia of Industrial Biotechnology. 2010. pp. 1–20. doi:10.1002/9780470054581.eib603. ISBN 978-0471799306.
- ↑ "The pharmacological basis and pathophysiological significance of the heart rate-lowering property of diltiazem". Fundamental & Clinical Pharmacology 13 (2): 145–153. 1999. doi:10.1111/j.1472-8206.1999.tb00333.x. PMID 10226758.
- ↑ "Drugs used in the management of heart disease and cardiac arrhythmias". Small Animal Clinical Pharmacology. Elsevier. 2008. pp. 380–457. doi:10.1016/b978-070202858-8.50019-1. ISBN 978-0-7020-2858-8.
- ↑ "[Calcium channel blockers and prevention of migraine]" (in fr). Pathologie-Biologie 40 (4): 381–388. April 1992. PMID 1353873.
- ↑ "Comparative clinical pharmacology of calcium channel blockers". American Family Physician 43 (2): 583–588. February 1991. PMID 1990741.
- ↑ "Beta-adrenoceptor blockers and calcium antagonists in the prophylaxis and treatment of migraine". Drugs 39 (3): 355–373. March 1990. doi:10.2165/00003495-199039030-00003. PMID 1970289.
- ↑ "[Evaluation of the effects of verapamil, flunarizine, diltiazem, nimodipine and placebo in the prevention of hemicrania. A double-blind randomized cross-over study]". La Clinica Terapeutica 134 (2): 119–125. July 1990. PMID 2147612.
- ↑ "Diltiazem prophylaxis in refractory migraine". The New England Journal of Medicine 310 (20): 1327–1328. May 1984. doi:10.1056/NEJM198405173102015. PMID 6144044.
- ↑ "The pharmacology of calcium channel antagonists: a novel class of anti-migraine agents?". Headache 23 (6): 278–283. November 1983. doi:10.1111/j.1526-4610.1983.hed2306278.x. PMID 6358127.
- ↑ Common Heart Drug May Reduce Cocaine Cravings. Sciencedaily.com (28 February 2008). Retrieved on 2012-10-21.
- ↑ "Augmented behavioral response and enhanced synaptosomal calcium transport induced by repeated cocaine administration are decreased by calcium channel blockers". Life Sciences 81 (7): 600–608. July 2007. doi:10.1016/j.lfs.2007.06.028. PMID 17689567.
- ↑ "Diltiazem enhances the analgesic but not the respiratory depressant effects of morphine in rhesus monkeys". European Journal of Pharmacology 397 (1): 85–92. May 2000. doi:10.1016/S0014-2999(00)00248-X. PMID 10844102.
- ↑ "Potentiation of analgesia and reversal of tolerance to morphine by calcium channel blockers". Indian Journal of Experimental Biology 39 (7): 636–642. July 2001. PMID 12019755.
- ↑ "A randomized trial of oral vs. topical diltiazem for chronic anal fissures". Diseases of the Colon and Rectum 44 (8): 1074–1078. August 2001. doi:10.1007/BF02234624. PMID 11535842.
- ↑ "The long-term results of diltiazem treatment for anal fissure". International Journal of Clinical Practice 60 (11): 1411–1413. November 2006. doi:10.1111/j.1742-1241.2006.00895.x. PMID 16911570.
- ↑ "The efficacy of diltiazem and glyceryltrinitrate for the medical management of chronic anal fissure: a meta-analysis". International Journal of Colorectal Disease 23 (1): 1–6. January 2008. doi:10.1007/s00384-007-0384-x. PMID 17846781.
External links
- "Diltiazem". Drug Information Portal. U.S. National Library of Medicine. https://druginfo.nlm.nih.gov/drugportal/name/diltiazem.
 |
