Chemistry:Mobocertinib
 | |
| Clinical data | |
|---|---|
| Trade names | Exkivity |
| Other names | TAK-788, AP-32788 |
| License data |
|
| Pregnancy category | |
| Routes of administration | By mouth |
| Drug class | Antineoplastic |
| ATC code | |
| Legal status | |
| Legal status | |
| Identifiers | |
| |
| CAS Number |
|
| PubChem CID | |
| DrugBank |
|
| ChemSpider | |
| UNII |
|
| KEGG | |
| ChEMBL | |
| Chemical and physical data | |
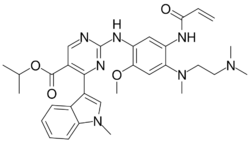
| Formula | C32H39N7O4 |
| Molar mass | 585.709 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
Mobocertinib, sold under the brand name Exkivity, is used for the treatment of non-small cell lung cancer.[5][7]
The most common side effects include diarrhea, rash, nausea, stomatitis, vomiting, decreased appetite, paronychia, fatigue, dry skin, and musculoskeletal pain.[5]
Mobocertinib is a small molecule tyrosine kinase inhibitor structurally similar to osimertinib (differs only by the presence of an additional isopropyl ester group).[8] Its molecular target is epidermal growth factor receptor (EGFR) bearing mutations in the exon 20 region.[9][10] Mobocertinib is an irreversible kinase inhibitor, forming a covalent bond with the cysteine 797 in the EGFR active site, leading to sustained inhibition of EGFR enzymatic activity. The irreversible binding leads to increased potency via higher affinity binding, more sustained EGFR kinase activity inhibition, and greater overall selectivity, as only a limited number of other kinases possess a cysteine in the equivalent position.[11]
Mobocertinib was approved for medical use in the United States in September 2021.[5][7] It is a first-in-class oral treatment to target EGFR Exon20 insertion mutations.[7]
Medical uses
Mobocertinib is indicated for adults with locally advanced or metastatic non-small cell lung cancer (NSCLC) with epidermal growth factor receptor (EGFR) exon 20 insertion mutations, as detected by an FDA-approved test, whose disease has progressed on or after platinum-based chemotherapy.[5]
Mechanism of action
Mobocertinib acts to inhibit EGFR exon 20 insertion mutations at a lower concentration than it does on wild-type proteins.[12]
Pharmacokinetics
The volume of distribution of Mobocertinib at steady state is 3,509 L.[12] The mean oral bioavailability of Mobocertinib is 37%.[12] The median Tmax is 4 hours.[12] The average half-life of Mobocertinib and its metabolites is 18 hours.[12] Mobocertinib is metabolized by CYP3A enzymes.[12]
Warnings
Mobocertinib may increase the chance of QTC prolongation, specifically Torsades de Pointes which can be fatal.[13]
Adverse Effects
More serious side effects of Mobocertinib may include agitation, bloating of the eyes, lips, feet, blurred vision, coma, decreased urine output, headache, hostility, diarrhea, depression, dizziness, fainting, lethargy, anxiety, nausea, seizures, weight gain, fatigue as well as edema.[13] Other side effects which may be less frequent are: chills, cough, dilated neck veins, ill-feeling and trouble with breathing.[13] Other notable side effects of taking Mobocertinib are: having an acidic stomach, heartburn, acidity, hair loss/thinning, bone pain, sore throat, stuffy nose, trouble swallowing, vomiting and weakness in hands and feet.[13]
History
Mobocertinib was studied in participants with previously treated metastatic non-small cell lung cancer with EGFR exon 20 insertions.[14][15]
The FDA granted the application for mobocertinib orphan drug designation.[16]
References
- ↑ 1.0 1.1 "Exkivity APMDS". 1 August 2022. https://www.tga.gov.au/resources/auspmd/exkivity.
- ↑ "Updates to the Prescribing Medicines in Pregnancy database". 21 December 2022. https://www.tga.gov.au/resources/resource/guidance/updates-prescribing-medicines-pregnancy-database.
- ↑ 3.0 3.1 "Exkivity- mobocertinib capsule". DailyMed. U.S. National Library of Medicine. https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=f1a91500-a944-4cb8-b4a8-ae278bcf728d.
- ↑ "Exkivity 40 mg hard capsules - Summary of Product Characteristics (SmPC)". 24 March 2022. https://www.medicines.org.uk/emc/product/13468/smpc.
- ↑ 5.0 5.1 5.2 5.3 5.4 "FDA grants accelerated approval to mobocertinib for metastatic non-small cell lung cancer with EGFR exon 20 insertion mutations". 16 September 2021. https://www.fda.gov/drugs/resources-information-approved-drugs/fda-grants-accelerated-approval-mobocertinib-metastatic-non-small-cell-lung-cancer-egfr-exon-20.
 This article incorporates text from this source, which is in the public domain.
This article incorporates text from this source, which is in the public domain.
- ↑ "Takedas Exkivity mobocertinib Receives Approval from the NMPA of China Becoming the First and Only Therapy Available for Patients with EGFR Exon20 Insertion+ NSCLC" (Press release). Takeda. 11 January 2023. Retrieved 10 February 2023.
- ↑ 7.0 7.1 7.2 "Takeda's Exkivity (mobocertinib) Approved by U.S. FDA as the First Oral Therapy Specifically Designed for Patients with EGFR Exon20 Insertion+ NSCLC" (Press release). Takeda Pharmaceutical Company. 15 September 2021. Archived from the original on 17 September 2021. Retrieved 16 September 2021 – via Business Wire.
- ↑ "Discovery of mobocertinib, a new irreversible tyrosine kinase inhibitor indicated for the treatment of non-small-cell lung cancer harboring EGFR exon 20 insertion mutations". Medicinal Chemistry Research 31 (10): 1647–1662. October 2022. doi:10.1007/s00044-022-02952-5. PMID 36065226.
- ↑ TAK-788 as First-line Treatment Versus Platinum-Based Chemotherapy for Non-Small Cell Lung Cancer (NSCLC) With EGFR Exon 20 Insertion Mutations. 28 January 2021. https://clinicaltrials.gov/ct2/show/NCT04129502?term=tak-788&phase=2&draw=2&rank=1. Retrieved 17 February 2021.
- ↑ "Spotlight on Mobocertinib (TAK-788) in NSCLC with EGFR Exon 20 Insertion Mutations". Lung Cancer: Targets and Therapy 12: 61–65. 2021. doi:10.2147/LCTT.S307321. PMID 34285620.
- ↑ "Mobocertinib (TAK-788): A Targeted Inhibitor of EGFR Exon 20 Insertion Mutants in Non-Small Cell Lung Cancer". Cancer Discovery 11 (7): 1672–1687. July 2021. doi:10.1158/2159-8290.CD-20-1683. PMID 33632773.
- ↑ 12.0 12.1 12.2 12.3 12.4 12.5 "Mobocertinib". https://go.drugbank.com/drugs/DB16390.
- ↑ 13.0 13.1 13.2 13.3 "Mobocertinib Side Effects: Common, Severe, Long Term" (in en). https://www.drugs.com/sfx/mobocertinib-side-effects.html.
- ↑ "Treatment Outcomes and Safety of Mobocertinib in Platinum-Pretreated Patients With EGFR Exon 20 Insertion-Positive Metastatic Non-Small Cell Lung Cancer: A Phase 1/2 Open-label Nonrandomized Clinical Trial". JAMA Oncology 7 (12): e214761. December 2021. doi:10.1001/jamaoncol.2021.4761. PMID 34647988.
- ↑ "Activity and Safety of Mobocertinib (TAK-788) in Previously Treated Non-Small Cell Lung Cancer with EGFR Exon 20 Insertion Mutations from a Phase I/II Trial". Cancer Discovery 11 (7): 1688–1699. July 2021. doi:10.1158/2159-8290.CD-20-1598. PMID 33632775.
- ↑ (PDF) Advancing Health Through Innovation: New Drug Therapy Approvals 2021 (Report). 13 May 2022. https://www.fda.gov/media/155227/download. Retrieved 22 January 2023.
 This article incorporates text from this source, which is in the public domain.
This article incorporates text from this source, which is in the public domain.
External links
- "Mobocertinib". Drug Information Portal. U.S. National Library of Medicine. https://druginfo.nlm.nih.gov/drugportal/name/mobocertinib.
- Clinical trial number NCT02716116 for "A Study of TAK-788 in Adults With Non-Small Cell Lung Cancer" at ClinicalTrials.gov
 |

