Chemistry:Propynal
From HandWiki
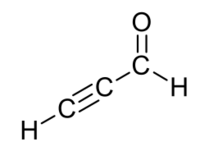
| |
| Names | |
|---|---|
| Preferred IUPAC name
Prop-2-ynal | |
| Other names
Propiolaldehyde; Propiolic aldehyde
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChemSpider | |
PubChem CID
|
|
| UNII | |
| |
| Properties | |
| C3H2O | |
| Molar mass | 54.048 g·mol−1 |
| Boiling point | 54–57 °C (129–135 °F; 327–330 K) |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Tracking categories (test):
Propynal is an organic compound with molecular formula HC2CHO. It is the simplest chemical compound containing both alkyne and aldehyde functional groups. It is a colorless liquid with explosive properties.[1]
The compound exhibits reactions expected for an electrophilic alkynyl aldehyde. It is a dienophile and a good Michael acceptor. Grignard reagents add to the carbonyl center.[1]
Occurrence in interstellar medium
Propynal has been observed in the interstellar medium. It is hypothesized to be formed from a carbon monoxide-acetylene complex.[2] Another possible pathway is through the reaction of propynylidyne (C3H) with water.[3]
Hazards
The compound is explosive, possibly because it tends to polymerize.[1]
See also
References
- ↑ 1.0 1.1 1.2 P. Perlmutter (2001). "Encyclopedia of Reagents for Organic Synthesis". Encyclopedia of Reagents for Organic Synthesis. doi:10.1002/047084289X.rp262m. ISBN 978-0471936237.
- ↑ Zhou, Li; Ralf I. Kaiser (2008), "Pathways to Oxygen-Bearing Molecules in the Interstellar Medium and in Planetary Atmospheres: Cyclopropenone (c-C3H2O) and Propynal (HCCCHO)", The Astrophysical Journal 686 (2), doi:10.1086/591072
- ↑ Xie, Hong-bin; Chang-bin Shao (2007), "Radical-Molecule Reaction C3H + H2O on Amorphous Water Ice: A Promising Route for Interstellar Propynal", The Astrophysical Journal 670 (1): 449–456, doi:10.1086/520757

