Chemistry:Methyl isocyanide
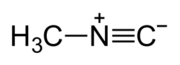
| |
| |
| Names | |
|---|---|
| IUPAC name
Isocyanomethane
| |
Other names
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChEBI | |
| ChemSpider | |
| DrugBank | |
| EC Number |
|
PubChem CID
|
|
| UNII | |
| |
| |
| Properties | |
| C2H3N | |
| Molar mass | 41.053 g·mol−1 |
| Appearance | Colorless liquid |
| Density | 0.69 g/mL |
| Melting point | −45 °C (−49 °F; 228 K) |
| Boiling point | 59 to 60 °C (138 to 140 °F; 332 to 333 K) |
| Miscible | |
| Hazards | |
| GHS pictograms |  
|
| GHS Signal word | Warning |
| H302, H312, H332, H373 | |
| P260, P261, P264, P270, P271, P280, P301+312, P302+352, P304+312, P304+340, P312, P314, P322, P330, P363, P501 | |
| NFPA 704 (fire diamond) | |
| Related compounds | |
Related compounds
|
acetic acid, acetamide, ethylamine, Acetonitrile |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
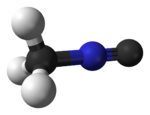
Methyl isocyanide or isocyanomethane is an organic compound and a member of the isocyanide family. This colorless liquid is isomeric and isoelectronic to methyl cyanide (acetonitrile), but its reactivity is very different. In contrast to the faintly sweet, ethereal odor of acetonitrile, the smell of methyl isocyanide, like that of other simple volatile isocyanides, is distinctly penetrating and vile. Methyl isocyanide is mainly used for making 5-membered heterocyclic rings. The C-N distance in methyl isocyanide is very short, 1.158 Å as is characteristic of isocyanides. [1]
Preparation and uses
Methyl isocyanide was first prepared by A. Gautier by reaction of silver cyanide with methyl iodide.[2][3] The common method for preparing methyl isocyanides is the dehydration of N-methylformamide.[4] Many metal cyanides react with methylating agents to give complexes of methyl isocyanide.[5] This kind of reactivity has been invoked as being relevant to the origin of life.[6]
Methyl isocyanide is useful for the preparation of diverse heterocycles. It is often used to prepare transition metal isocyanide complexes.[7]
Safety
Methyl isocyanide is very endothermic (ΔfH⦵(g) = +150.2 kJ/mol, 3.66 kJ/g) and can isomerize explosively to acetonitrile.[8] A sample exploded when heated in a sealed ampoule, and during redistillation at 59 °C/1 bar, a drop of liquid fell back into the dry boiler flask and exploded violently. The explosive decomposition of methyl isocyanide has been studied in detail.[9]
References
- ↑ Kessler, Myer; Ring, Harold; Trambarulo, Ralph; Gordy, Walter (1950-07-01). "Microwave Spectra and Molecular Structures of Methyl Cyanide and Methyl Isocyanide". Physical Review (American Physical Society (APS)) 79 (1): 54–56. doi:10.1103/physrev.79.54. ISSN 0031-899X. Bibcode: 1950PhRv...79...54K.
- ↑ Gautier, A. (1868). "Ueber eine neue Reihe von Verbindungen, welche mit den Cyanwasserstoffsäure-Aethern isomer sind". Justus Liebigs Annalen der Chemie 146 (1): 119–124. doi:10.1002/jlac.18681460107. https://zenodo.org/record/1427279.
- ↑ Gautier, A. (1869). "Des Nitriles des Acides Gras: Deuxième Partie - Des Carbylamines". Annales de Chimie et de Physique 17: 203. http://gallica.bnf.fr/ark:/12148/bpt6k34827s/f102.image.langEN.
- ↑ R. E. Schuster, James E. Scott, and Joseph Casanova, Jr (1966). "Methyl isocyanide". Organic Syntheses 46: 75. doi:10.15227/orgsyn.046.0075.
- ↑ Fehlhammer, Wolf P.; Fritz, Marcus. (1993). "Emergence of a CNH and Cyano Complex Based Organometallic Chemistry". Chemical Reviews 93 (3): 1243–1280. doi:10.1021/cr00019a016.
- ↑ Mariani, Angelica; Russell, David; Javelle, Thomas; Sutherland, John (2018). "A Light-Releasable Potentially Prebiotic Nucleotide Activating Agent". Journal of the American Chemical Society 140 (28): 8657–8661. doi:10.1021/jacs.8b05189. PMID 29965757.
- ↑ Eckert, H.; Nestl, A.; Ugi, I. (2001). "Methyl Isocyanide". Encyclopedia of Reagents for Organic Synthesis. John Wiley & Sons. doi:10.1002/047084289X.rm198. ISBN 0471936235.
- ↑ Clothier, P. Q. E.; Glionna, M. T. J.; Pritchard, H. O. (July 1985). "Thermal explosions of methyl isocyanide in spherical vessels". The Journal of Physical Chemistry 89 (14): 2992–2996. doi:10.1021/j100260a008. ISSN 0022-3654. https://pubs.acs.org/doi/pdf/10.1021/j100260a008.
- ↑ Urben, Peter (22 May 2017) (in en). Bretherick's Handbook of Reactive Chemical Hazards | ScienceDirect. ISBN 9780081009710. https://www.sciencedirect.com/book/9780081009710/brethericks-handbook-of-reactive-chemical-hazards. Retrieved 2022-02-23.
External links
 |



