Chemistry:Silmitasertib
 | |
| Clinical data | |
|---|---|
| Other names | CX-4945 |
| ATC code |
|
| Pharmacokinetic data | |
| Bioavailability | Orally bioavailable |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| ChEMBL | |
| PDB ligand | |
| Chemical and physical data | |
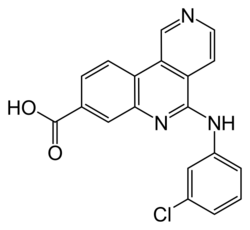
| Formula | C19H12ClN3O2 |
| Molar mass | 349.77 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
Silmitasertib (INN), codenamed CX-4945, is a small-molecule inhibitor of protein kinase CK2 (casein kinase II), a constitutively active serine/threonine-specific protein kinase that is overexpressed in several types of tumors.
Silmitasertib is in clinical trials for use as an adjunct to chemotherapy in the treatment of cholangiocarcinoma (bile duct cancer),[1] is in phase I and II clinical trials for the treatment of recurrent Sonic Hedgehog (SHH) medulloblastoma,[2][3] and in preclinical development for other cancers, including hematological and lymphoid malignancies.[4]
In January 2017, it was granted orphan drug status by the U.S. Food and Drug Administration for advanced cholangiocarcinoma. It is being developed by Senhwa Biosciences of Taiwan.[5]
Mechanism of action
Silmitasertib interacts competitively with the ATP-binding site of CK2 subunit alpha. This leads to inhibition of several downstream signaling pathways, including PI3K/Akt.[6][7]
COVID-19 infections
In SARS-CoV-2 (COVID-19) infected Caco-2 cells, the phosphorylase activity of casein kinase 2 (CK2) is increased resulting in phosphorylation of several cytoskeletal proteins. These infected cells also display CK2-containing filopodia protrusions associated with budding viral particles. Hence the protrusions may assist the virus in infecting adjacent cells. In these same cells, the CK2 inhibitor silmitasertib displayed potent antiviral activity.[8] Senhwa Biosciences and the US National Institutes of Health have announced that they will evaluate the efficacy of silmitasertib in treating COVID-19 infections.[9]
History
CX-4945 was originated by now-defunct Cylene Pharmaceuticals of San Diego, California, as the culmination of a lengthy process of rational, structure-based molecular modification of a lead compound known to have PARP inhibitor activity.[10] Among a large series of compounds built around a benzo[c]-[2,6]naphthyridine-8-carboxylic acid scaffold, CX-4945 was chosen for its high potency and selectivity as an inhibitor of CK2.[10]
Preclinical pharmacokinetics studies conducted in mice, rats, and dogs confirmed that CX-4945 had satisfactory bioavailability when given by mouth and did not block cytochrome P450, while experiments in mice confirmed its inhibition of solid-tumor growth in a dose-dependent manner.[7][10]
Clinical trials in humans began in 2010, making CK-4945 the first CK2 inhibitor to reach this stage of drug development.[7][10] The International Nonproprietary Name silmitasertib was proposed in 2010 and recommended by the World Health Organization in 2011.[11][12]
References
- ↑ "Owner". 31 October 2022. https://www.americanlifefund.com/financial-assistance-for-bile-duct-cancer-patients/.
- ↑ Clinical trial number NCT03904862 for "PBTC-053: A Pediatric Brain Tumor Consortium Phase I/ II and Surgical Study of CX-4945 in Patients With Recurrent SHH Medulloblastoma" at ClinicalTrials.gov
- ↑ "Developmental phosphoproteomics identifies the kinase CK2 as a driver of Hedgehog signaling and a therapeutic target in medulloblastoma". Science Signaling 11 (547): eaau5147. September 2018. doi:10.1126/scisignal.aau5147. PMID 30206138.
- ↑ "Casein Kinase II (CK2) as a Therapeutic Target for Hematological Malignancies". Current Pharmaceutical Design 23 (1): 95–107. 2017. doi:10.2174/1381612822666161006154311. PMID 27719640.
- ↑ "CX-4945 granted orphan drug designation". Oncology Times 39 (5): 23. 2017. doi:10.1097/01.COT.0000514203.35081.69.
- ↑ "The casein kinase 2 inhibitor, CX-4945, as an anti-cancer drug in treatment of human hematological malignancies". Frontiers in Pharmacology 6: 70. 2015. doi:10.3389/fphar.2015.00070. PMID 25873900.
- ↑ 7.0 7.1 7.2 "CX-4945, an orally bioavailable selective inhibitor of protein kinase CK2, inhibits prosurvival and angiogenic signaling and exhibits antitumor efficacy". Cancer Research 70 (24): 10288–10298. December 2010. doi:10.1158/0008-5472.CAN-10-1893. PMID 21159648.
- ↑ "The Global Phosphorylation Landscape of SARS-CoV-2 Infection". Cell 182 (3): 685–712.e19. August 2020. doi:10.1016/j.cell.2020.06.034. PMID 32645325.
- ↑ "Senhwa Biosciences, NIH to co-develop COVID-19 drug". BioSpectrum. 27 April 2020. https://www.biospectrumasia.com/news/34/15848/senhwa-biosciences-nih-to-co-develop-covid-19-drug.html.
- ↑ 10.0 10.1 10.2 10.3 "Discovery and SAR of 5-(3-chlorophenylamino)benzo[c][2,6]naphthyridine-8-carboxylic acid (CX-4945), the first clinical stage inhibitor of protein kinase CK2 for the treatment of cancer". Journal of Medicinal Chemistry 54 (2): 635–654. January 2011. doi:10.1021/jm101251q. PMID 21174434.
- ↑ "International Nonproprietary Names for Pharmaceutical Substances (INN). Proposed International Nonproprietary Names: List 103". WHO Drug Information 24 (2): 166–167. 2010. http://apps.who.int/iris/bitstream/10665/74553/1/24_2_2010_INN103.pdf.
- ↑ "International Nonproprietary Names for Pharmaceutical Substances (INN). Recommended International Nonproprietary Names: List 66". WHO Drug Information 25 (3): 329. 2011. https://www.who.int/medicines/publications/druginformation/innlists/RL66_final.pdf.
 |
