Chemistry:Sodium hydroselenide
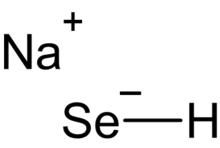
| |
| Names | |
|---|---|
| IUPAC name
Sodium hydroselenide
| |
| Other names
Sodium biselenide
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChemSpider | |
PubChem CID
|
|
| |
| |
| Properties | |
| NaSeH | |
| Molar mass | 102.969 g·mol−1 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Sodium hydroselenide is an inorganic compound with the chemical formula NaSeH. It is a salt of hydrogen selenide. It consist of sodium cations Na+
and hydroselenide anions −
SeH. Each unit consists of one sodium, one selenium, and one hydrogen atom. Sodium hydroselenide is a selenium analog of sodium hydroxide NaOH.
Production
Sodium hydroselenide can be made by reducing selenium with sodium borohydride:
Alternatively it can be made from sodium ethoxide exposed to hydrogen selenide:[1]
- CH
3CH
2O−
Na+
+ H
2Se → NaSeH + CH
3CH
2OH
Sodium hydroselenide is not made for storage, instead it is used immediately after production in a fume hood thanks to the appalling odour of hydrogen selenide.
Properties
Sodium hydroselenide dissolves in water or ethanol. In humid air sodium hydroselenide is changed to sodium polyselenide and elemental selenium.[1]
Sodium hydroselenide is slightly reducing.[1]
Use
In organic synthesis, hydrogen sodium hydroselenide is a nucleophillic agent for insertion of selenium.[1]
References
- ↑ 1.0 1.1 1.2 1.3 Młochowski, Jacek; Syper, Ludwik (2001). "Sodium Hydrogen Selenide". Encyclopedia of Reagents for Organic Synthesis. doi:10.1002/047084289X.rs079. ISBN 0471936235.
 |

