Chemistry:Temgicoluril
 | |
| Clinical data | |
|---|---|
| Trade names | Adaptol, Mebicar |
| Other names | Adaptol; Mebicar; Mebicarum; Mebikar; Tetramethylglycoluril; 1,3,4,6-Tetramethylglycoluril |
| ATC code | |
| Legal status | |
| Legal status |
|
| Pharmacokinetic data | |
| Metabolism | Gastrointestinal tract: 77–80% |
| Elimination half-life | 3 hours[1] |
| Excretion | Urine: 55–70% |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| Chemical and physical data | |
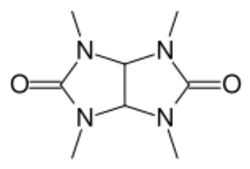
| Formula | C8H14N4O2 |
| Molar mass | 198.226 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
Temgicoluril (INN),[2] also known as tetramethylglycoluril and sold under the brand names Adaptol and Mebicar, is an anxiolytic medication produced by Latvian pharmaceutical company Olainfarm and sold in Latvia and Russia .[3]
The chemical structure of temgicoluril is somewhat similar to uric acid and it doesn't interact with acids, alkali, oxidants and reducing agents. It seems to affect all major neurotransmitter systems.[4]
Temgicoluril has an effect on the structure of limbic–reticular activity, particularly on hypothalamus emotional zone, as well as on all several basic neuromediator systems – γ aminobutyric acid (GABA), choline, serotonin, and adrenergic activity. It decreases brain norepinephrine levels and increases brain serotonin levels without modulating dopaminergic systems or cholinergic systems.[5]
Temgicoluril purportedly has anti-anxiety (anxiolytic) properties.[5][6][7][8][9] It is also used to aid smoking cessation.[3] In addition, temgicoluril may be useful in the treatment of ADHD symptoms.[10] In contrast with typical anxiolytic medications such as benzodiazepines, temgicoluril is non-habit forming, non-sedating, and does not impair motor function.[6][3]
It can be prepared by condensation of N,N-dimethylurea with glyoxal. One publication reported an elegant procedure for doing this. They combined N,N-dimethylurea and glyoxal with a catalytic amount of phosphoric acid anhydride in an aqueous solution at room temperature and after sufficient time temgicoluril was conveniently isolated by filtration. The filtrate can be re-used by adding more dimethylurea and glyoxal (no additional catalyst is needed) and obtaining respectable yields, although this requires a longer reaction time.[11]
As of 2021, temgicoluril has not been evaluated outside of Latvia and Russia.
Medical uses
Temgicoluril is used in Latvia and Russia, as a pharmaceutical drug to treat anxiety and to prevent or reduce anxiety, unrest, fear, internal emotional tension and irritability, reduce neuroses and neurotic disorders, heartburns of non-coronary heart disease origin. These effects are not accompanied with relaxation of muscle tone and impaired coordination of movement, suppression of mental and physical activity, so the drug can be used without interruption of work or school.
Temgicoluril does not have a direct effect on sleep, however, it enhances the effectiveness of sleep medicines and normalizes the course of disturbed sleep. Temgicoluril alleviates or eliminates the manifestations of nicotine dependence that occur after smoking cessation. Temgicoluril does not cause mood swings or euphoria, no habituation and addiction, withdrawal syndrome has been observed.
Side effects
Possible and rare side effects may include dizziness, hypotension, indigestion, allergic reactions (itchy skin) after high doses, hypothermia, fatigue. And lowered blood pressure and/or body temperature decreased by 1 to 1.5 °C. Blood pressure and body temperature return to normal after completion of treatment.[12]
See also
- Fabomotizole
- Phenibut
- Selank
- Validol
- Bemethyl
References
- ↑ Schwarz J, Weisspapir M, "Sustained release pharmaceutical composition containing mebicar", US patent 20110070305, published 2011-03-24, assigned to Alpharx Inc.
- ↑ "International Nonproprietary Names for Pharmaceutical Substances (INN)". WHO Drug Information 34 (3). 2020. https://cdn.who.int/media/docs/default-source/international-nonproprietary-names-(inn)/pl124.pdf?sfvrsn=6437f035_10&download=true.
- ↑ 3.0 3.1 3.2 "Adaptol product summary". JSC Olainfarm. Latvia. http://olainfarm.lv/en/prescription-drugs/adaptol-3.
- ↑ "Clinical Overview ADAPTOL". JSC Olainfarm. Latvia. https://www.farmagalenica.it/wp-content/uploads/2017/10/Mebicar-clinical-overview.pdf.
- ↑ 5.0 5.1 "[Characteristics of the psychotropic spectrum of action of mebicar]". Biulleten' Eksperimental'noi Biologii I Meditsiny 89 (5): 568–570. May 1980. PMID 6104993.
- ↑ 6.0 6.1 "A study of the spectrum of psychotropic action of mebicar". Bulletin of Experimental Biology and Medicine 89 (5): 621–624. 1980. doi:10.1007/BF00835799.
- ↑ "[Adaptol--verges of possible]". Likars'ka Sprava (5): 125–133. 2012. PMID 23534281.
- ↑ "[Generalized anxiety disorder: psychosomatic aspects and treatment approaches]". Zhurnal Nevrologii I Psikhiatrii Imeni S.S. Korsakova 112 (1): 40–44. 2012. PMID 22678674.
- ↑ "[Asthenic disorders in children]". Zhurnal Nevrologii I Psikhiatrii Imeni S.S. Korsakova 110 (11 Pt 1): 26–29. 2010. PMID 21183919.
- ↑ "[Adaptol in the treatment of ADHD]". Zhurnal Nevrologii I Psikhiatrii Imeni S.S. Korsakova 109 (8): 45–48. 2009. PMID 19738569.
- ↑ "A green synthesis of glycoluril derivatives in aqueous solution with recycle of the waste". Green Chemistry Letters and Reviews 6 (2): 135–139. 2013-06-01. doi:10.1080/17518253.2012.718803. ISSN 1751-8253. Bibcode: 2013GCLR....6..135M.
- ↑ "Adaptol (Mebicarum) - Summary of product characteristics" (in lv). https://www.zva.gov.lv/zvais/zalu-registrs/en/info/03-0002.
 |

