Chemistry:Tandospirone
 | |
| Clinical data | |
|---|---|
| Trade names | Sediel |
| Other names | Metanopirone |
| AHFS/Drugs.com | International Drug Names |
| Routes of administration | Oral |
| ATC code |
|
| Legal status | |
| Legal status |
|
| Pharmacokinetic data | |
| Metabolites | 1-PP |
| Elimination half-life | Tandospirone: 2–3 hours 1-PP: 3–5 hours |
| Excretion | Urine (70%; 0.1% as unchanged drug) |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| IUPHAR/BPS | |
| ChemSpider | |
| UNII | |
| ChEBI | |
| ChEMBL | |
| Chemical and physical data | |
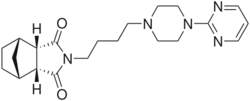
| Formula | C21H29N5O2 |
| Molar mass | 383.496 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| | |
Tandospirone, sold under the brand name Sediel, is an anxiolytic and antidepressant medication used in Japan and China , where it is marketed by Dainippon Sumitomo Pharma. It is a member of the azapirone class of drugs and is closely related to other azapirones like buspirone and gepirone.
Tandospirone was introduced for medical use in Japan in 1996[1] and in China in 2004.[2]
Medical uses
Anxiety and depression
Tandospirone is most commonly used as a treatment for anxiety and depressive disorders, such as generalised anxiety disorder and dysthymia respectively.[3] For both indications it usually takes a couple of weeks for therapeutic effects to begin to be seen,[3] although at higher doses more rapid anxiolytic responses have been seen.[4] It has also been used successfully as a treatment for bruxism.[5]
Augmentation for depression
Tandospirone can be used as an effective augmentation,[clarification needed] especially when coupled with fluoxetine or clomipramine.[6]
Other uses
Tandospirone has been tried successfully as an adjunctive treatment for cognitive symptoms[clarification needed] in schizophrenic individuals.[7]
Side effects
Common adverse effects include:[3][1]
- Dizziness
- Drowsiness
- Insomnia
- Headache
- Gastrointestinal disorders
- Dry mouth
- Negative influence on explicit memory function[3]
- Nausea[1]
Adverse effects with unknown frequency include:[3]
- Hypotension (low blood pressure)
- Dysphoria
- Tachycardia
- Malaise
- Psychomotor impairment
It is not believed to be addictive but is known to produce mild withdrawal effects (e.g., anorexia) after abrupt discontinuation.[3]
Pharmacology
Pharmacodynamics
Tandospirone acts as a potent and selective 5-HT1A receptor partial agonist, with a Ki affinity value of 27 ± 5 nM[8] and approximately 55 to 85% intrinsic activity.[9][10] It has relatively weak affinity for the 5-HT2A (1,300 ± 200), 5-HT2C (2,600 ± 60), α1-adrenergic (1,600 ± 80), α2-adrenergic (1,900 ± 400), D1 (41,000 ± 10,000), and D2 (1,700 ± 300) receptors, and is essentially inactive at the 5-HT1B, 5-HT1D, β-adrenergic, and muscarinic acetylcholine receptors, serotonin transporter, and benzodiazepine allosteric site of the GABAA receptor (all of which are > 100,000).[8] There is evidence of tandospirone having low but significant antagonistic activity at the α2-adrenergic receptor through its active metabolite 1-(2-pyrimidinyl)piperazine (1-PP).[11][12]
Chemistry
Synthesis
- The Noreximide [6319-06-8] precursor also has dual uses to make Taglutimide & Tripamide & Lurasidone.

The catalytic hydrogenation of cis-5-Norbornene-exo-2,3-dicarboxylic anhydride [129-64-6] (1) gives Norbornane-2exo,3exo-dicarboxylic Acid-anhydride [14166-28-0] (2). Reaction with aqueous ammonia leads to Exo-2,3-norbornanedicarboximide [14805-29-9] (3). Alkylation with 1,4-dibromobutane [110-52-1] (4) gives CID:10661911 (5). Alkylation of the remaining halogen with 2-(1-Piperazinyl)Pyrimidine [20980-22-7] (6) completed the synthesis of Tandospirone (7).
History
Tandospirone was introduced in Japan for the treatment of anxiety disorders in 1996.[1] It was subsequently also introduced in China in 2004.[2]
Society and culture
Name
Tandospirone is also known as metanopirone and by the developmental code name SM-3997.[18][19][20][5] It is marketed in Japan under the brand name Sediel.[18][19][20][5]
References
- ↑ 1.0 1.1 1.2 1.3 "The 5-HT1A receptor: an unkept promise". Anxiolytics. Milestones in Drug Therapy. Birkhäuser Basel. 2012. p. 99. ISBN 978-3-0348-8470-9. https://books.google.com/books?id=JZW-BwAAQBAJ&pg=PA99. Retrieved 2023-10-07.
- ↑ 2.0 2.1 NeuroPsychopharmacotherapy. Springer International Publishing. 2022. p. 2131. ISBN 978-3-030-62059-2. https://books.google.com/books?id=G1qaEAAAQBAJ&pg=PA2131. Retrieved 2023-10-07.
- ↑ 3.0 3.1 3.2 3.3 3.4 3.5 "Tandospirone". CNS Drugs 5 (2): 147–153. February 1996. doi:10.2165/00023210-199605020-00006.
- ↑ "Tandospirone in the treatment of generalised anxiety disorder and mixed anxiety-depression : results of a comparatively high dosage trial". Clinical Drug Investigation 24 (2): 121–126. 2004. doi:10.2165/00044011-200424020-00007. PMID 17516698.
- ↑ 5.0 5.1 5.2 "Tandospirone". Martindale: The Complete Drug Reference. The Royal Pharmaceutical Society of Great Britain. 23 September 2011. http://www.medicinescomplete.com/mc/martindale/current/10442-w.htm. Retrieved 14 November 2013.
- ↑ "Role of tandospirone, a 5-HT1A receptor partial agonist, in the treatment of central nervous system disorders and the underlying mechanisms". Oncotarget (Impact Journals, LLC) 8 (60): 102705–102720. November 2017. doi:10.18632/oncotarget.22170. PMID 29254282.
- ↑ "Enhancement of cognitive performance in schizophrenia by addition of tandospirone to neuroleptic treatment". The American Journal of Psychiatry 158 (10): 1722–1725. October 2001. doi:10.1176/appi.ajp.158.10.1722. PMID 11579010.
- ↑ 8.0 8.1 "Analysis of tandospirone (SM-3997) interactions with neurotransmitter receptor binding sites". Biological Psychiatry 28 (2): 99–109. July 1990. doi:10.1016/0006-3223(90)90627-E. PMID 1974152.
- ↑ "Effects of tandospirone on second messenger systems and neurotransmitter release in the rat brain". General Pharmacology 26 (8): 1765–1772. December 1995. doi:10.1016/0306-3623(95)00077-1. PMID 8745167.
- ↑ "Effects of tandospirone, a novel anxiolytic agent, on human 5-HT1A receptors expressed in Chinese hamster ovary cells (CHO cells)". Biogenic Amines 18 (3): 319–328. 2004. doi:10.1163/1569391041501933.
- ↑ "Tandospirone and its metabolite, 1-(2-pyrimidinyl)-piperazine--II. Effects of acute administration of 1-PP and long-term administration of tandospirone on noradrenergic neurotransmission". Neuropharmacology 30 (7): 691–701. July 1991. doi:10.1016/0028-3908(91)90176-C. PMID 1681447.
- ↑ "Kinetics, brain uptake, and receptor binding of tandospirone and its metabolite 1-(2-pyrimidinyl)-piperazine". Journal of Clinical Psychopharmacology 12 (5): 341–345. October 1992. doi:10.1097/00004714-199210000-00009. PMID 1362206.
- ↑ "Synthesis and anxiolytic activity of N-substituted cyclic imides (1R*,2S*,3R*,4S*)-N-[4-[4-(2-pyrimidinyl)-1-piperazinyl]butyl]-2,3- bicyclo[2.2.1]heptanedicarboximide (tandospirone) and related compounds". Chemical & Pharmaceutical Bulletin 39 (9): 2288–2300. September 1991. doi:10.1248/cpb.39.2288. PMID 1687114.
- ↑ "An Efficient Synthesis of Buspirone and its Analogues.". Archiv der Pharmazie 325 (5): 313–315. 1992. doi:10.1002/ardp.19923250513. ISSN 0365-6233.
- ↑ "SM-3997". Drugs of the Future 11 (11): 949. 1986. doi:10.1358/dof.1986.011.11.53048.
- ↑ "14C-labeling of a novel anxiolytic agent tandospirone". Journal of Labelled Compounds and Radiopharmaceuticals 31 (6): 427–436. June 1992. doi:10.1002/jlcr.2580310602. ISSN 0362-4803.
- ↑ Hansen JB, Thomsen MS, WO patent 2012016569, assigned to Conrig Pharma ApS
- ↑ 18.0 18.1 The Dictionary of Drugs: Chemical Data: Chemical Data, Structures and Bibliographies. Springer US. 2014. p. 1149. ISBN 978-1-4757-2085-3. https://books.google.com/books?id=0vXTBwAAQBAJ&pg=PA1149. Retrieved 2023-10-07.
- ↑ 19.0 19.1 Schweizerischer Apotheker-Verein (2004). Index Nominum: International Drug Directory. Medpharm Scientific Publishers. p. 1146. ISBN 978-3-88763-101-7. https://books.google.com/books?id=EgeuA47Ocm4C&pg=PA1146. Retrieved 2023-10-07.
- ↑ 20.0 20.1 Concise Dictionary of Pharmacological Agents: Properties and Synonyms. Springer Netherlands. 2012. p. 257. ISBN 978-94-011-4439-1. https://books.google.com/books?id=tsjrCAAAQBAJ&pg=PA257. Retrieved 2023-10-07.
 |

