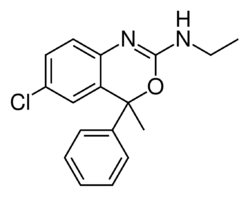
Chemistry:Etifoxine
 | |
 | |
| Clinical data | |
|---|---|
| Trade names | Stresam |
| Other names | Étifoxine; Etifoxin; Etafenoxine; Etafenoxin; EFX; Hoe 36801; Hoe-36,801 |
| AHFS/Drugs.com | International Drug Names |
| Pregnancy category |
|
| Routes of administration | Oral administration[2] |
| ATC code | |
| Legal status | |
| Legal status |
|
| Pharmacokinetic data | |
| Bioavailability | 90%[3] |
| Protein binding | 88–95%[4] |
| Metabolism | Liver[5] |
| Metabolites | Several (including diethyletifoxine)[5] |
| Elimination half-life | Etifoxine: 6 hours[5] Diethyletifoxine: 20 hours[5] |
| Excretion | Mainly urine, also bile[5][2] |
| Identifiers | |
| |
| CAS Number |
|
| PubChem CID | |
| IUPHAR/BPS | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEMBL | |
| Chemical and physical data | |
| Formula | C17H17ClN2O |
| Molar mass | 300.79 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| (verify) | |
Etifoxine, sold under the trade name Stresam among others, is a nonbenzodiazepine anxiolytic agent, primarily indicated for short-term management of adjustment disorder, specifically instances of situational depression accompanied by anxiety, such as stress-induced anxiety.[2][6] Administration is by mouth.[7] Side effects associated with etifoxine use include slight drowsiness, headache, skin eruptions, and allergic reactions.[2][8][9] In rare cases, etifoxine has been linked to severe skin and liver toxicity, as well as menstrual bleeding between periods.[8][1] Unlike benzodiazepines, etifoxine does not cause sedation or lack of coordination.[10][3] Etifoxine acts as a GABAA receptor positive allosteric modulator and as a ligand for translocator proteins.[10] Both mechanisms are conjectured to contribute to its anxiolytic properties.[10][3]
Etifoxine was developed in the 1960s and was introduced for medical use in France in 1979.[11] Its marketed in 53 countries worldwide, although it remains unavailable in the United States.[7][11][12] Throughout the 2010s and early 2020s, the safety profile of etifoxine was scrutinized within France and the European Union, prompted by reports of toxicity.[13][8][7] The investigation revealed that instances of toxicity were infrequent, and etifoxine was allowed to remain on the market.[13][8][7]
Medical uses
Etifoxine has historically been used in the treatment of "psychosomatic manifestations of anxiety", for instance "autonomic dystonia, particularly with cardiovascular expression".[7][13][8][1] Subsequently, the indication for etifoxine has been more formalized as treatment of adjustment disorder (situational depression) with anxiety (ADWA) (e.g., stress-related anxiety).[7][14][3] Etifoxine has been found to reduce scores on the Hamilton Anxiety Rating Scale (HAM-A) in people with adjustment disorder with anxiety by approximately 50 to 75% after 4 weeks of treatment in clinical trials (e.g., AMETIS, ETILOR, ETIZAL, STRETI studies).[7] The medication is similarly effective or more effective than benzodiazepines like lorazepam, alprazolam, and clonazepam and more effective than buspirone for adjustment disorder with anxiety on the basis of directly comparative randomized controlled trials.[14][3][15][16][17][4] However, in the AMETIS study, both etifoxine and lorazepam failed to show greater effectiveness over placebo.[7]
The usual dosage of etifoxine (as the hydrochloride salt) is 150 to 200 mg per day in divided doses of 50 to 100 mg two to three times per day (e.g., 50 mg–50 mg–100 mg).[2][7][6][18][1][19][20] It is taken for a few days to a few weeks, but no longer than 12 weeks.[2][13][7][5]
Available forms
Etifoxine is provided in the form of oral capsules containing 50 mg etifoxine hydrochloride.[2][1][21][22]
Contraindications
Etifoxine is contraindicated in people with circulatory shock, severe liver impairment, severe kidney impairment, myasthenia gravis, galactosemia (due to lactose in the drug formulation), severe respiratory failure, and hypersensitivity (allergy) to etifoxine.[2][5] The medication is not recommended in children or adolescents under the age of 18[5] and is not recommended during pregnancy and breastfeeding due to insufficient data.[2][1] Caution is warranted with regard to combining etifoxine and other central depressants such as benzodiazepines, central analgesics, antipsychotics, sedative antihistamines, and alcohol.[2][1]
Side effects
Side effects of etifoxine include slight drowsiness and headache.[2][9] Rarely, etifoxine can cause benign skin eruptions or rashes and allergic reactions such as hives and angioedema.[2][8][1] Etifoxine shows less adverse effects of anterograde amnesia, sedation, impaired psychomotor performance, and withdrawal syndromes than those of benzodiazepines.[5] No cases of misuse or dependence with etifoxine were identified in a French pharmacovigilance survey, which is also in contrast to benzodiazepines.[8]
Etifoxine has been associated rarely with cases of severe dermal toxicity and liver toxicity.[8][23] Skin and subcutaneous disorders are the most frequently reported, but these generally resolve after drug cessation.[3] A 2012 review of etifoxine by the French National Pharmacovigilance Committee determined that etifoxine was safe and continued to provide a favorable alternative to benzodiazepine anxiolytics. The committee found (for a ten-year pharmacovigilance period) that safety concerns were rare or very rare and that the incidence of idiosyncratic hepatic (liver) problems were very rare.[13]
Pharmacology
Pharmacodynamics
Unlike benzodiazepines, etifoxine may produce its anxiolytic effects through a dual mechanism, by directly binding to GABAA receptors and (purportedly, exact binding site undetermined) to the mitochondrial translocator protein (TSPO). This results in stimulation of the biosynthesis of endogenous neurosteroids, for instance allopregnanolone, a highly potent GABAA receptor positive allosteric modulator.[24]
At GABAA receptors etifoxine binds at the α+β− interface and preferentially potentiates α2β3γ2 and α3β3γ2 receptor types.[25] This direct allosteric potentiation can only be observed at relatively high concentrations (starting at >1 mM) and is perhaps not physiologically relevant at normal human doses.[26] This is different from benzodiazepines and etifoxine can be used alongside benzodiazepines to potentiate their effects without competing for binding sites;[27] however, it also means that the direct effects of etifoxine are not reversed by the benzodiazepine antagonist flumazenil.[28]
Pharmacokinetics
Etifoxine is taken via oral administration.[2][5] It is rapidly absorbed from the gastrointestinal tract.[5] It is well-absorbed, with a bioavailability of 90%.[2][3] The time to peak levels of etifoxine is 2 to 3 hours.[5] The plasma protein binding of etifoxine is 88 to 95%.[4] It does not bind to blood cells.[2] The drug is known to cross the placental barrier.[2] Etifoxine is metabolized in the liver into several metabolites.[5] One of these metabolites, diethyletifoxine, is pharmacologically active.[5] The elimination half-life of etifoxine is 6 hours and of diethyletifoxine is almost 20 hours.[5] Etifoxine is eliminated in three phases.[2] The drug is excreted mainly in urine in the form of metabolites.[5] It is also excreted in bile.[5] Only small amounts are excreted unchanged.[5]
Chemistry
Etifoxine is a nonbenzodiazepine—that is, it is similarly a GABAA receptor positive allosteric modulator but its chemical structure is distinct from that of benzodiazepines.[3][29] Instead, it is a benzoxazine derivative.[3]
Etifoxine is used pharmaceutically as the hydrochloride salt.[30][31]
(S)-Etifoxine, the (S) enantiomer of etifoxine, was under development by Anvyl Pharmaceuticals for the treatment of neuropathic pain, but development was discontinued.[32] A deuterated form of etifoxine with improved pharmacokinetics known as deuterated etifoxine (GRX-917) is under development by GABA Therapeutics for the treatment anxiety and mood disorders.[33][34][24][35]
History
Etifoxine was developed by Hoechst in the 1960s.[11][36] It was introduced for medical use in France in 1979.[11] Since at least 2000, etifoxine has been marketed by the French pharmaceutical company Biocodex.[31][29][37][19] Following reports of post-marketing toxicity, the safety of etifoxine was reassessed by the French government[13][8] and the European Medicines Agency (EMA).[38][39] In January 2022, the EMA "finalized its review of Stresam and concluded that the medicine can continue to be used for the treatment of anxiety disorders, but it must not be used in patients who previously had severe skin reactions or severe liver problems after taking etifoxine."[38][39]
Society and culture
Names
Etifoxine is the generic name of the drug and its INN, BAN, and DCF.[30][31] It is also known by the older and much-lesser-used synonym etafenoxine[40] and by its developmental code name Hoe 36801.[30][31] Etifoxine is marketed under the brand name Stresam.[30][31][19] It has also been marketed under the brand name Strezam, specifically in Russia.[19]
Availability
Etifoxine has been marketed in 53 countries as of 2022.[7][11] Some of the countries in which etifoxine has been marketed include Argentina , Bulgaria, Chile , France , Luxembourg, Malta, Romania, Russia , South Africa , Thailand, and Ukraine .[19][13][7][31] Etifoxine is not approved for use by the United States Food and Drug Administration (FDA) or the European Medicines Agency (EMA) of the European Union, and hence is not marketed in these regions.[11][7] However, etifoxine is marketed in four European Union member states (Bulgaria, Luxembourg, Malta, Romania).[13][7]
See also
References
- ↑ 1.0 1.1 1.2 1.3 1.4 1.5 1.6 1.7 Afect (1 May 2011). Traité de chimie thérapeutique Volume 7: Médicaments actifs sur le système nerveux central. Lavoisier. pp. 500–. ISBN 978-2-7430-1373-8. OCLC 758328876. https://books.google.com/books?id=IgMK7O4XSxQC&pg=PA500. "5.2. Propriétés pharmacologiques. [...] 5.2.2. Étifoxine. Utilisé dans les manifestations psychosomatiques de l'anxiété, telles que les dystonies neurovégétatives (Stresam, gélules à 50 mg). La posologie usuelle est de 150 à 200 mg/j. 5.4. Effets indésirables. [...] 5.4.2. Étifoxine. Légère somnolence en début de traitement, éruptions cutanées rares. 5.5. Contre-indications et précautions d'emploi. [...] 5.5.2. Étifoxine. Précaution lors d'association avec les dépresseurs centraux (benzodiazepines, analgesiques centraux, neuroleptiques, antihistaminiques H1 sédatifs, etc.). L'alcool potentialise l'effet sédatif de l'étifoxine. Ce produit est déconseillé pendant la grossesse et en cas d'allaitement."
- ↑ 2.00 2.01 2.02 2.03 2.04 2.05 2.06 2.07 2.08 2.09 2.10 2.11 2.12 2.13 2.14 2.15 2.16 "STRESAM®, capsule Summary of Product Characteristics". GABA Therapeutics, Inc. July 2010. https://gabarx.com/wp-content/uploads/2018/05/StresamSPC-1.pdf.
- ↑ 3.0 3.1 3.2 3.3 3.4 3.5 3.6 3.7 3.8 "An update on the anxiolytic and neuroprotective properties of etifoxine: from brain GABA modulation to a whole-body mode of action". Neuropsychiatr Dis Treat 15: 1781–1795. 2019. doi:10.2147/NDT.S200568. PMID 31308671.
- ↑ 4.0 4.1 4.2 "Etifoxine is non-inferior than clonazepam for reduction of anxiety symptoms in the treatment of anxiety disorders: a randomized, double blind, non-inferiority trial". Psychopharmacology (Berl) 237 (11): 3357–3367. November 2020. doi:10.1007/s00213-020-05617-6. PMID 33009629.
- ↑ 5.00 5.01 5.02 5.03 5.04 5.05 5.06 5.07 5.08 5.09 5.10 5.11 5.12 5.13 5.14 5.15 5.16 5.17 "Etifoxine for pain patients with anxiety". Korean J Pain 28 (1): 4–10. January 2015. doi:10.3344/kjp.2015.28.1.4. PMID 25589941.
- ↑ 6.0 6.1 "Étifoxine : études cliniques récentes" (in fr). L'Encéphale 34 (Suppl 1 (Etifoxine : un nouveau regard sur le récepteur GABA et l'anxiété)): S9–S14. January 2008. doi:10.1016/S0013-7006(08)71386-1. ISSN 0013-7006.
- ↑ 7.00 7.01 7.02 7.03 7.04 7.05 7.06 7.07 7.08 7.09 7.10 7.11 7.12 7.13 "INN/active substance: etifoxine Assessment report". European Medicines Agency. 27 January 2022. https://gabarx.com/wp-content/uploads/2022/06/etifoxine-containing-medicinal-products-article-31-referral-public-assessment-report-including_en.pdf.
- ↑ 8.0 8.1 8.2 8.3 8.4 8.5 8.6 8.7 8.8 "Safety profile of etifoxine: A French pharmacovigilance survey". Fundam Clin Pharmacol 30 (2): 147–52. April 2016. doi:10.1111/fcp.12169. PMID 26588183.
- ↑ 9.0 9.1 "Etifoxine impairs neither alertness nor cognitive functions of the elderly: A randomized, double-blind, placebo-controlled crossover study". Eur Neuropsychopharmacol 28 (8): 925–932. August 2018. doi:10.1016/j.euroneuro.2018.05.011. PMID 30135030.
- ↑ 10.0 10.1 10.2 "Anxiolytics targeting GABAA receptors: Insights on etifoxine". World J Biol Psychiatry 19 (sup1): S36–S45. 2018. doi:10.1080/15622975.2018.1468030. PMID 30204559.
- ↑ 11.0 11.1 11.2 11.3 11.4 11.5 "Novel pharmacological targets in drug development for the treatment of anxiety and anxiety-related disorders". Pharmacol Ther 204: 107402. December 2019. doi:10.1016/j.pharmthera.2019.107402. PMID 31470029.
- ↑ "Hermitage Man Sentenced for Importing and Selling Drugs Not Approved by FDA" (in en). 2019-10-07. https://www.justice.gov/usao-wdpa/pr/hermitage-man-sentenced-importing-and-selling-drugs-not-approved-fda.
- ↑ 13.0 13.1 13.2 13.3 13.4 13.5 13.6 13.7 "COMMISSION NATIONALE DE PHARMACOVIGILANCE Compte rendu de la réunion du mardi 26 juin 2012" (in fr). http://ansm.sante.fr/var/ansm_site/storage/original/application/56a2e1cb1dbc986720da09842df11c22.pdf.
- ↑ 14.0 14.1 "Pharmacotherapy of adjustment disorder: A review". World J Biol Psychiatry 19 (sup1): S46–S52. 2018. doi:10.1080/15622975.2018.1492736. PMID 30204560.
- ↑ "Traitement du trouble de l'adaptation avec anxiété: évaluation de l'efficacité et de la tolérance de l'étifoxine par un essai en double aveugle contre produit de référence" (in French). Encephale 24 (6): 569–74. 1998. PMID 9949940.
- ↑ "Efficacy of etifoxine compared to lorazepam monotherapy in the treatment of patients with adjustment disorders with anxiety: a double-blind controlled study in general practice". Hum Psychopharmacol 21 (3): 139–49. April 2006. doi:10.1002/hup.757. PMID 16625522.
- ↑ "Etifoxine versus alprazolam for the treatment of adjustment disorder with anxiety: a randomized controlled trial". Adv Ther 32 (1): 57–68. January 2015. doi:10.1007/s12325-015-0176-6. PMID 25620535.
- ↑ "L'étifoxine: un nouveau regard sur le récepteur GABA et l'anxiété" (in French). Encephale 34 Spec No 1: 1–11. April 2008. doi:10.1016/S0013-7006(08)70553-0. ISSN 0013-7006. PMID 18826172.
- ↑ 19.0 19.1 19.2 19.3 19.4 "Micromedex Products: Please Login". https://www.micromedexsolutions.com/micromedex2/librarian/.
- ↑ "A double blind parallel group placebo controlled comparison of sedative and mnesic effects of etifoxine and lorazepam in healthy subjects [corrected]". Fundam Clin Pharmacol 15 (3): 209–16. June 2001. doi:10.1046/j.1472-8206.2001.00025.x. PMID 11468032.
- ↑ Concours Médical, Volume 123, Issues 34-40. 2001. p. 2361. OCLC 1564649. https://books.google.com/books?id=c-IjAQAAMAAJ. "STRESAM gélule . COMPOSITION - Etifoxine chlorhydrate , 50 mg par gélule . Excipients q.s.p. 1 géluie de 200 mg . INDICATIONS THÉRAPEUTIQUES - Manifestations psychosomatiques de l'anxiété telles que dystonies neurovégétatives [...]"
- ↑ Le préparateur en pharmacie - Guide théorique et pratique (2e ed.). Lavoisier. 15 April 2013. pp. 820–. ISBN 978-2-7430-6371-9. OCLC 1005722892. https://books.google.com/books?id=W12vAgAAQBAJ&pg=PA820. "L'étifoxine (Stresam®, gél 50 mg, liste I) a son indication dans les manifestations psychosomatiques de l'anxiété telles que dystonies neurovégétatives, notamment à expression cardiovasculaire."
- ↑ "Etifoxine-induced acute hepatitis: a case series". Clin Res Hepatol Gastroenterol 36 (5): e85–8. October 2012. doi:10.1016/j.clinre.2012.04.002. PMID 22633197.
- ↑ 24.0 24.1 "Neurosteroids and translocator protein 18 kDa (TSPO) in depression: implications for synaptic plasticity, cognition, and treatment options". Eur Arch Psychiatry Clin Neurosci 273 (7): 1477–1487. December 2022. doi:10.1007/s00406-022-01532-3. PMID 36574032.
- ↑ "Involvement of the GABAA receptor α subunit in the mode of action of etifoxine". Pharmacol. Res. 145: 104250. 2019. doi:10.1016/j.phrs.2019.04.034. PMID 31059790. https://hal.archives-ouvertes.fr/hal-02344585/file/Mattei%20et%20al-Pharmacol%20Res%202019.pdf.
- ↑ "The modulatory effects of etifoxine's direct effects on GABA(A) receptors are mediated by the beta subunit". Neuropharmacology 45 (3): 293–303. 2003. doi:10.1016/s0028-3908(03)00187-4. PMID 12871647.
- ↑ "Potentiation of clobazam's anticonvulsant activity by etifoxine, a non-benzodiazepine tranquilizer, in mice. Comparison studies with sodium valproate". Arzneimittelforschung 36 (9): 1320–2. 1986. PMID 3098254.
- ↑ "Interactions of etifoxine with the chloride channel coupled to the GABA(A) receptor complex". NeuroReport 10 (15): 3207–10. 1999. doi:10.1097/00001756-199910190-00015. PMID 10574561.
- ↑ 29.0 29.1 "Axonal regeneration and neuroinflammation: roles for the translocator protein 18 kDa". J Neuroendocrinol 24 (1): 71–81. January 2012. doi:10.1111/j.1365-2826.2011.02215.x. PMID 21951109.
- ↑ 30.0 30.1 30.2 30.3 The Dictionary of Drugs: Chemical Data: Chemical Data, Structures and Bibliographies. Springer. 14 November 2014. pp. 523–. ISBN 978-1-4757-2085-3. OCLC 1058412474. https://books.google.com/books?id=0vXTBwAAQBAJ&pg=PA523.
- ↑ 31.0 31.1 31.2 31.3 31.4 31.5 Swiss Pharmaceutical Society (2000). Index Nominum 2000: International Drug Directory. Taylor & Francis. pp. 416–. ISBN 978-3-88763-075-1. https://books.google.com/books?id=5GpcTQD_L2oC&pg=PA416.
- ↑ "S-Etifoxine - AdisInsight". https://adisinsight.springer.com/drugs/800047916.
- ↑ "Etifoxine deuterated - GABA Therapeutics - AdisInsight". https://adisinsight.springer.com/drugs/800056063.
- ↑ "Translocator protein (18kDa) TSPO: a new diagnostic or therapeutic target for stress-related disorders?". Mol Psychiatry 27 (7): 2918–2926. July 2022. doi:10.1038/s41380-022-01561-3. PMID 35444254. https://epub.uni-regensburg.de/52300/1/s41380-022-01561-3.pdf.
- ↑ "The imidazodiazepine, KRM-II-81: An example of a newly emerging generation of GABAkines for neurological and psychiatric disorders". Pharmacol Biochem Behav 213: 173321. February 2022. doi:10.1016/j.pbb.2021.173321. PMID 35041859.
- ↑ U.S. Patent 3,725,404
- ↑ "An update into the medicinal chemistry of translocator protein (TSPO) ligands". Eur J Med Chem 209: 112924. January 2021. doi:10.1016/j.ejmech.2020.112924. PMID 33081988.
- ↑ 38.0 38.1 "Etifoxine-containing medicinal products". European Medicines Agency. 27 January 2022. https://www.ema.europa.eu/en/medicines/human/referrals/etifoxine-containing-medicinal-products.
- ↑ 39.0 39.1 "EU OKs Continued Use Of Etifoxine For Anxiety Despite Toxicity Concerns". Pink Sheet Informa Pharma Intelligence. January 31, 2022. https://pink.pharmaintelligence.informa.com/PS145610/EU-OKs-Continued-Use-Of-Etifoxine-For-Anxiety-Despite-Toxicity-Concerns.
- ↑ "Translocator protein (TSPO) ligands for the diagnosis or treatment of neurodegenerative diseases: a patent review (2010 - 2015; part 2)". Expert Opin Ther Pat 26 (11): 1353–1366. November 2016. doi:10.1080/13543776.2016.1230605. PMID 27599163.
Further reading
- "Étifoxine : pharmacologie comportementale" (in fr). L'Encéphale 34 (Suppl 1 (Etifoxine : un nouveau regard sur le récepteur GABA et l'anxiété)): S15–S19. January 2008. doi:10.1016/S0013-7006(08)71387-3. ISSN 0013-7006.
- "Étifoxine et circuits des émotions" (in fr). L'Encéphale 34 (Suppl 1 (Etifoxine : un nouveau regard sur le récepteur GABA et l'anxiété)): S21–S27. January 2008. doi:10.1016/S0013-7006(08)71388-5. ISSN 0013-7006.
- "Étifoxine et récepteurs GABA" (in fr). L'Encéphale 34 (Suppl 1 (Etifoxine : un nouveau regard sur le récepteur GABA et l'anxiété)): S29–S34. January 2008. doi:10.1016/S0013-7006(08)71389-7. ISSN 0013-7006.
- "Étifoxine, neurostéroïdes et anxiété" (in fr). L'Encéphale 34 (Suppl 1 (Etifoxine : un nouveau regard sur le récepteur GABA et l'anxiété)): S35–S43. January 2008. doi:10.1016/S0013-7006(08)71390-3. ISSN 0013-7006.
- "L'étifoxine: un nouveau regard sur le récepteur GABA et l'anxiété" (in French). Encephale 34 Spec No 1: 1–11. April 2008. doi:10.1016/S0013-7006(08)70553-0. ISSN 0013-7006. PMID 18826172.
- "Translocator protein (18 kDa) as a target for novel anxiolytics with a favourable side-effect profile". J Neuroendocrinol 24 (1): 82–92. January 2012. doi:10.1111/j.1365-2826.2011.02166.x. PMID 21609361.
- "Axonal regeneration and neuroinflammation: roles for the translocator protein 18 kDa". J Neuroendocrinol 24 (1): 71–81. January 2012. doi:10.1111/j.1365-2826.2011.02215.x. PMID 21951109.
- "Analgesic strategies aimed at stimulating the endogenous production of allopregnanolone". Front Cell Neurosci 8: 174. 2014. doi:10.3389/fncel.2014.00174. PMID 24987335.
- "Etifoxine for pain patients with anxiety". Korean J Pain 28 (1): 4–10. January 2015. doi:10.3344/kjp.2015.28.1.4. PMID 25589941.
- "Safety profile of etifoxine: A French pharmacovigilance survey". Fundam Clin Pharmacol 30 (2): 147–52. April 2016. doi:10.1111/fcp.12169. PMID 26588183.
- "Anxiolytics targeting GABAA receptors: Insights on etifoxine". World J Biol Psychiatry 19 (sup1): S36–S45. 2018. doi:10.1080/15622975.2018.1468030. PMID 30204559.
- "Pharmacotherapy of adjustment disorder: A review". World J Biol Psychiatry 19 (sup1): S46–S52. 2018. doi:10.1080/15622975.2018.1492736. PMID 30204560.
- "An update on the anxiolytic and neuroprotective properties of etifoxine: from brain GABA modulation to a whole-body mode of action". Neuropsychiatr Dis Treat 15: 1781–1795. 2019. doi:10.2147/NDT.S200568. PMID 31308671.
- "Diagnostic and Therapeutic Potential of TSPO Studies Regarding Neurodegenerative Diseases, Psychiatric Disorders, Alcohol Use Disorders, Traumatic Brain Injury, and Stroke: An Update". Cells 9 (4): 870. April 2020. doi:10.3390/cells9040870. PMID 32252470.
- "TSPO: an emerging role in appetite for a therapeutically promising biomarker". Open Biol 11 (8): 210173. August 2021. doi:10.1098/rsob.210173. PMID 34343461.
- "Neurosteroids and translocator protein 18 kDa (TSPO) in depression: implications for synaptic plasticity, cognition, and treatment options". Eur Arch Psychiatry Clin Neurosci 273 (7): 1477–1487. December 2022. doi:10.1007/s00406-022-01532-3. PMID 36574032.
External links
- Stesam (etifoxine hydrochloride) Summary of Product Characteristics (SPC)
- Stresam (etifoxine hydrochloride) Patient Leaflet
- Stresam (etifoxine hydrochloride) Package Insert
- Etifoxine French Commission Nationale de Pharmacovigilance Review (Original French)
- Etifoxine French Commission Nationale de Pharmacovigilance Review (English Translation)
- Etifoxine European Medicines Agency Assessment Report
 |

