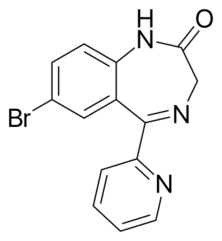
Chemistry:Bromazepam
 | |
 | |
| Clinical data | |
|---|---|
| Trade names | Lexotan, Lexotanil, others |
| AHFS/Drugs.com | Micromedex Detailed Consumer Information |
| Routes of administration | By mouth |
| ATC code | |
| Legal status | |
| Legal status |
|
| Pharmacokinetic data | |
| Bioavailability | 84% |
| Metabolism | Liver |
| Elimination half-life | 12–20 hours (avg. 17hr)[1] |
| Excretion | Kidney |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEBI | |
| ChEMBL | |
| Chemical and physical data | |
| Formula | C14H10BrN3O |
| Molar mass | 316.158 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| (verify) | |
Bromazepam, sold under many brand names, is a benzodiazepine. It is mainly an anti-anxiety agent with similar side effects to diazepam. In addition to being used to treat anxiety or panic states, bromazepam may be used as a premedicant prior to minor surgery. Bromazepam typically comes in doses of 3 mg and 6 mg tablets.[2]
It was patented in 1961 by Roche and approved for medical use in 1974.[3]
Medical uses
Medical uses include treatment of severe anxiety.[4] Despite certain side effects and the emergence of alternative products (e.g. pregabalin), benzodiazepine medication remains an effective way of reducing problematic symptoms, and is typically deemed effective by patients[5][6] and medical professionals.[7][8][9] Similarly to other intermediate-acting depressants, it may be used as hypnotic medication[10] or in order to mitigate withdrawal effects of alcohol consumption.[11][12][13]
Pharmacology

Bromazepam is a "classical" benzodiazepine; other classical benzodiazepines include: diazepam, clonazepam, oxazepam, lorazepam, nitrazepam, flurazepam, and clorazepate.[14] Its molecular structure is composed of a diazepine connected to a benzene ring and a pyridine ring, the benzene ring having a single nitrogen atom that replaces one of the carbon atoms in the ring structure.[15] It is a 1,4-benzodiazepine, which means that the nitrogens on the seven-sided diazepine ring are in the 1 and 4 positions.
Bromazepam binds to the GABA receptor GABAA, causing a conformational change and increasing the inhibitory effects of GABA. It acts as a positive modulator, increasing the receptors' response when activated by GABA itself or an agonist (such as alcohol). As opposed to barbital, BZDs are not GABA-receptor activators and rely on increasing the neurotransmitter's natural activity.[16] Bromazepam is an intermediate-acting benzodiazepine, is moderately lipophilic compared to other substances of its class[17] and metabolised hepatically via oxidative pathways.[18] It does not possess any antidepressant or antipsychotic qualities.[19]
After night time administration of bromazepam a highly significant reduction of gastric acid secretion occurs during sleep followed by a highly significant rebound in gastric acid production the following day.[20]
Bromazepam alters the electrical status of the brain causing an increase in beta activity and a decrease in alpha activity in EEG recordings.[21]
Pharmacokinetics
Bromazepam is reported to be metabolized by a hepatic enzyme belonging to the Cytochrome P450 family of enzymes. In 2003, a team led by Oda Manami at Oita Medical University reported that CYP3A4, a member of the Cytochrome P450 family, was not the responsible enzyme since itraconazole, a known inhibitor of CYP3A4, did not affect its metabolism.[22] In 1995, J. van Harten at the Solvay Pharmaceutical Department of Clinical Pharmacology in Weesp reported that fluvoxamine, which is a potent inhibitor of CYP1A2, a less potent CYP3A4 inhibitor, and a negligible inhibitor of CYP2D6, does inhibit its metabolism.[23]
The major metabolite of bromazepam is hydroxybromazepam,[22] which is an active agent too and has a half-life approximately equal to that of bromazepam.[citation needed]
Side-effects
Bromazepam is similar in side effects to other benzodiazepines. The most common side effects reported are drowsiness, sedation, ataxia, memory impairment, and dizziness.[24] Impairments to memory functions are common with bromazepam and include a reduced working memory and reduced ability to process environmental information.[25][26][27] A 1975 experiment on healthy, male college students exploring the effects of four different drugs on learning capacity observed that taking bromazepam alone at 6 mg 3 times daily for 2 weeks impaired learning capacities significantly. In combination with alcohol, impairments in learning capacity became even more pronounced.[28] Various studies report impaired memory, visual information processing and sensory data and impaired psychomotor performance;[29][30][31] deterioration of cognition including attention capacity and impaired co-ordinative skills;[32][33] impaired reactive and attention performance, which can impair driving skills;[34] drowsiness and decrease in libido.[35][36] Unsteadiness after taking bromazepam is, however, less pronounced than other benzodiazepines such as lorazepam.[37]
On occasion, benzodiazepines can induce extreme alterations in memory such as anterograde amnesia and amnesic automatism, which may have medico-legal consequences. Such reactions occur usually only at the higher dose end of the prescribing spectrum.[38]
Very rarely, dystonia can develop.[39]
Up to 30% treated on a long-term basis develop a form of dependence, i.e. these patients cannot stop the medication without experiencing physical and/or psychological benzodiazepine withdrawal symptoms.
Leukopenia and liver-damage of the cholestatic type with or without jaundice (icterus) have additionally been seen; the original manufacturer Roche recommends regular laboratory examinations to be performed routinely.
Ambulatory patients should be warned that bromazepam may impair the ability to drive vehicles and to operate machinery. The impairment is worsened by consumption of alcohol, because both act as central nervous system depressants. During the course of therapy, tolerance to the sedative effect usually develops.
Frequency and seriousness of adverse effects
As with all medication, the frequency and seriousness of side-effects varies greatly depending on quantities consumed.[40][41] In a study about bromazepam's negative effects on psychomotor skills and driving ability, it was noted that 3 mg doses caused minimal impairment.[42] It also appeared that impairment may be tied to methods of testing more so than on the product's intrinsic activity.[43]
Moreover, side-effects other than drowsiness, dizziness and ataxia seem to be rare[44] and not experienced by more than a few percent of users. The use of other, comparable medication seems to display an identically moderate side-effect profile.[45][46][47]
Tolerance, dependence and withdrawal
Prolonged use of bromazepam can cause tolerance and may lead to both physical and psychological dependence on the drug, and as a result, it is a medication which is controlled by international law. It is nonetheless important to note that dependence, long-term use and misuse occur in a minority of cases[48][49][50] and are not representative of most patients' experience with this type of medication.[51][52]
It shares with other benzodiazepines the risk of abuse, misuse, psychological dependence or physical dependence.[53][54] A withdrawal study demonstrated both psychological dependence and physical dependence on bromazepam including marked rebound anxiety after 4 weeks chronic use. Those whose dose was gradually reduced experienced no withdrawal.[55]
Patients treated with bromazepam for generalised anxiety disorder were found to experience withdrawal symptoms such as a worsening of anxiety, as well as the development of physical withdrawal symptoms when abruptly withdrawn bromazepam.[56] Abrupt or over rapid withdrawal from bromazepam after chronic use even at therapeutic prescribed doses can lead to a severe withdrawal syndrome including status epilepticus and a condition resembling delerium tremens.[57][58][59]
Animal studies have shown that chronic administration of diazepam (or bromazepam) causes a decrease in spontaneous locomotor activity, decreased turnover of noradrenaline and dopamine and serotonin, increased activity of tyrosine hydroxylase and increased levels of the catecholamines. During withdrawal of bromazepam or diazepam a fall in tryptophan, serotonin levels occurs as part of the benzodiazepine withdrawal syndrome.[60] Changes in the levels of these chemicals in the brain can cause headaches, anxiety, tension, depression, insomnia, restlessness, confusion, irritability, sweating, dysphoria, dizziness, derealization, depersonalization, numbness/tingling of extremities, hypersensitivity to light, sound, and smell, perceptual distortions, nausea, vomiting, diarrhea, appetite loss, hallucinations, delirium, seizures, tremor, stomach cramps, myalgia, agitation, palpitations, tachycardia, panic attacks, short-term memory loss, and hyperthermia.[61][62]
Overdose
Bromazepam is commonly involved in drug overdoses.[63] A severe bromazepam benzodiazepine overdose may result in an alpha pattern coma type.[64] The toxicity of bromazepam in overdosage increases when combined with other CNS depressant drugs such as alcohol or sedative hypnotic drugs.[65] Similarly to other benzodiazepines however, being a positive modulator of certain neuroreceptors and not an agonist, the product has reduced overdose potential compared to older products of the barbiturate class. Its consumption alone is very seldom fatal in healthy adults.[66][67]
Bromazepam was in 2005 the most common benzodiazepine involved in intentional overdoses in France .[68] Bromazepam has also been responsible for accidental poisonings in companion animals. A review of benzodiazepine poisonings in cats and dogs from 1991 to 1994 found bromazepam to be responsible for significantly more poisonings than any other benzodiazepine.[69]
Contraindications
Benzodiazepines require special precaution if used in elderly, pregnant, child, alcohol- or drug-dependent individuals and individuals with comorbid psychiatric disorders.[70]
Special populations
- Globally, bromazepam is contraindicated and should be used with caution in women who are pregnant, the elderly, patients with a history of alcohol or other substance abuse disorders and children.
- In 1987, a team of scientists led by Ochs reported that the elimination half-life, peak serum concentration, and serum free fraction are significantly elevated and the oral clearance and volume of distribution significantly lowered in elderly subjects.[71] The clinical consequence is that the elderly should be treated with lower doses than younger patients.
- Bromazepam may affect driving and ability to operate machinery.[72]
- Bromazepam is pregnancy category D, a classification that means that bromazepam has been shown to cause harm to the unborn child. The Hoffman LaRoche product information leaflet warns against breast feeding while taking bromazepam. There has been at least one report of sudden infant death syndrome linked to breast feeding while consuming bromazepam.[73][74]
Interactions
Cimetidine, fluvoxamine and propranolol causes a marked increase in the elimination half-life of bromazepam leading to increased accumulation of bromazepam.[71][75][23]
Society and culture
Drug misuse
Bromazepam has a similar misuse risk as other benzodiazepines such as diazepam.[76] In France car accidents involving psychotropic drugs in combination with alcohol (itself a major contributor) found benzodiazepines, mainly diazepam, nordiazepam, and bromazepam, to be the most common drug present in the blood stream, almost twice that of the next-most-common drug cannabis.[77] Bromazepam has also been used in serious criminal offences including robbery, homicide, and sexual assault.[78][79][80]
Brand names
It is marketed under several brand names, including, Brozam, Lectopam, Lexomil, Lexotan, Lexilium, Lexaurin, Brazepam, Rekotnil, Bromaze, Somalium, Lexatin, Calmepam, Zepam and Lexotanil.[81]
Legal status
Bromazepam is a Schedule IV drug under the Convention on Psychotropic Substances.[82]
Synthesis

See also
References
- ↑ "Lexotan (bromazepam) Product Insert". Roche. 23 October 2012. http://e-lactancia.org/media/papers/Bromazepam-DS-2012.pdf.
- ↑ "Bromazepam". Pharmaceutical Benefits Scheme (PBS). Australian Government - Department of Health. http://www.pbs.gov.au/medicine/item/4150K-4151L.
- ↑ (in en) Analogue-based Drug Discovery. John Wiley & Sons. 2006. p. 53X. ISBN 9783527607495. https://books.google.com/books?id=FjKfqkaKkAAC&pg=PA53X.
- ↑ "Content Not Available". https://www.uptodate.com/contents/bromazepam-drug-information?source=search_result&search=bromazepam&selectedTitle=1~3.
- ↑ "[Bromazepam in the treatment of somatized psychogenic disorders (author's transl)]" (in German). Wiener Klinische Wochenschrift 91 (7): 240–244. March 1979. PMID 34934.
- ↑ "[A clinical trial of bromazepam (author's transl)]" (in French). La Nouvelle Presse Médicale 11 (22): 1699–1701. May 1982. PMID 6124936.
- ↑ "[Efficacy and tolerance of alprazolam and bromazepam in flexible doses. Double-blind study in 119 ambulatory anxious patients]" (in French). L'Encéphale 13 (2): 89–95. 1987. PMID 2885173.
- ↑ "Bromazepam: acute benefit-risk assessment in general practice". Current Medical Research and Opinion 8 (10): 683–688. 11 August 2008. doi:10.1185/03007998409110117. PMID 6144455.
- ↑ "Bromazépam" (in French). Haute Autorité de santé (HAS). 7 September 2016. https://www.has-sante.fr/upload/docs/evamed/CT-15448_LEXOMIL_PIS_RI_avis2_CT15448.pdf.
- ↑ "Bromazepam". http://flipper.diff.org/app/items/info/5074.
- ↑ "Bromazépam". Répertoire des Spécialités Pharmaceutiques. ANSM: Agence Nationale de Sécurité du Médicament et des Produits de Santé (French: National Security Agency of Medicines and Health Products). http://agence-prd.ansm.sante.fr/php/ecodex/frames.php?specid=67851467&typedoc=R&ref=R0157296.htm.
- ↑ "Hypnotic action of benzodiazepines: a possible mechanism". Life Sciences 34 (18): 1763–1768. April 1984. doi:10.1016/0024-3205(84)90576-9. PMID 6145073.
- ↑ "A multi-centre, double-blind parallel trial of bromazepam ('Lexotan') and lorazepam to compare the acute benefit-risk ratio in the treatment of patients with anxiety". Current Medical Research and Opinion 9 (7): 505–510. 11 August 2008. doi:10.1185/03007998509109625. PMID 2863089.
- ↑ "Pharmacological characterization of benzodiazepine receptors in the brain". European Journal of Pharmacology 48 (3): 263–270. April 1978. doi:10.1016/0014-2999(78)90085-7. PMID 639854.
- ↑ Bromazepam Eutimia.com - Salud Mental. © 1999-2002.
- ↑ "Anxiolytics targeting GABAA receptors: Insights on etifoxine". The World Journal of Biological Psychiatry 19 (sup1): S36–S45. 11 September 2018. doi:10.1080/15622975.2018.1468030. PMID 30204559.
- ↑ "Correlation of lipophilicity descriptors with pharmacokinetic parameters of selected benzodiazepines". African Journal of Biomedical Research 19 (3): 213–218. 25 November 2016. https://www.ajol.info/index.php/ajbr/article/view/150280.
- ↑ "[Chemical and pharmacologic aspects of benzodiazepines]" (in German). Schweizerische Rundschau für Medizin Praxis 78 (27–28): 766–772. July 1989. PMID 2570451.
- ↑ "[The action of bromazepam on anxiety (author's transl)]" (in French). La Nouvelle Presse Médicale 11 (22): 1738–1740. May 1982. PMID 6124947.
- ↑ "Inhibitory effect of bromazepam on basal and betazole-stimulated gastric acid secretion in man". Gut 15 (2): 116–120. February 1974. doi:10.1136/gut.15.2.116. PMID 4820635.
- ↑ "Blood levels and electroencephalographic effects of diazepam and bromazepam". Clinical Pharmacology and Therapeutics 20 (2): 184–191. August 1976. doi:10.1002/cpt1976202184. PMID 7375.
- ↑ 22.0 22.1 "The effect of itraconazole on the pharmacokinetics and pharmacodynamics of bromazepam in healthy volunteers". European Journal of Clinical Pharmacology 59 (8–9): 615–619. November 2003. doi:10.1007/s00228-003-0681-4. PMID 14517708.
- ↑ 23.0 23.1 "Overview of the pharmacokinetics of fluvoxamine". Clinical Pharmacokinetics 29 (Suppl 1): 1–9. 1995. doi:10.2165/00003088-199500291-00003. PMID 8846617.
- ↑ "LECTOPAM®". RxMed. http://www.rxmed.com/b.main/b2.pharmaceutical/b2.1.monographs/CPS-%20Monographs/CPS-%20%28General%20Monographs-%20L%29/LECTOPAM.html.
- ↑ "Effects of alprazolam and bromazepam on visual search and verbal recognition memory in humans: a study with event-related brain potentials". Neuropsychobiology 34 (1): 49–56. 1996. doi:10.1159/000119291. PMID 8884760.
- ↑ "[Neuromodulatory effects of caffeine and bromazepam on visual event-related potential (P300): a comparative study]". Arquivos de Neuro-Psiquiatria 63 (2B): 410–415. June 2005. doi:10.1590/s0004-282x2005000300009. PMID 16059590.
- ↑ "Responsiveness of sensorimotor cortex during pharmacological intervention with bromazepam". Neuroscience Letters 448 (1): 33–36. December 2008. doi:10.1016/j.neulet.2008.10.024. PMID 18938214.
- ↑ "Effect of two weeks' treatment with thioridazine, chlorpromazine, sulpiride and bromazepam, alone or in combination with alcohol, on learning and memory in man". Psychopharmacologia 44 (2): 205–208. October 1975. doi:10.1007/BF00421011. PMID 710.
- ↑ "Gastric acid secretion, serum-gastrin levels and psychomotor function under the influence of placebo, insulin-hypoglycemia, and/or bromazepam". International Journal of Clinical Pharmacology and Biopharmacy 13 (1): 1–10. January 1976. PMID 2560.
- ↑ "Effects of single oral doses of bromazepam, buspirone and clobazam on performance tasks and memory". Neuropsychobiology 22 (3): 141–145. 1989. doi:10.1159/000118609. PMID 2577220.
- ↑ "The effects of bromazepam on the early stage of visual information processing (P100)". Arquivos de Neuro-Psiquiatria 65 (4A): 955–959. December 2007. doi:10.1590/s0004-282x2007000600006. PMID 18094853.
- ↑ "Psychomotor skills during subacute treatment with thioridazine and bromazepam, and their combined effects with alcohol". Annals of Clinical Research 8 (2): 117–123. April 1976. PMID 7178.
- ↑ "Acute effects of bromazepam on signal detection performance, digit symbol substitution test and smooth pursuit eye movements". Neuropsychobiology 20 (2): 91–95. 1988. doi:10.1159/000118481. PMID 2908134.
- ↑ "Two Weeks' Treatment with Chlorpromazine, Thioridazine, Sulpiride, or Bromazepam: Actions and Interactions with Alcohol on Psychomotor Skills Related to Driving". Alcohol, Drugs and Driving. Modern Trends in Pharmacopsychiatry. 11. 1976. pp. 85–90. doi:10.1159/000399456. ISBN 978-3-8055-2349-3.
- ↑ "[Clinical trial of bromazepam. Thirty-four cases (author's transl)]" (in French). La Nouvelle Presse Médicale 11 (22): 1741–1743. May 1982. PMID 6124948.
- ↑ "[Bromazepam in anxiety. Clinical evaluation (author's transl)]" (in French). La Nouvelle Presse Médicale 11 (22): 1722–1724. May 1982. PMID 6124942.
- ↑ "Effect on postural sway of various benzodiazepine tranquillizers". British Journal of Clinical Pharmacology 20 (1): 9–16. July 1985. doi:10.1111/j.1365-2125.1985.tb02792.x. PMID 2862898.
- ↑ "[Memory gaps and hypercomplex automatisms after a single oral dose of benzodiazepines: clinical and medico-legal aspects]" (in French). Annales Médico-Psychologiques 144 (1): 102–109. January 1986. PMID 2876672.
- ↑ "Bromazepam-induced dystonia". Biomedicine & Pharmacotherapy 46 (8): 375–376. January 1992. doi:10.1016/0753-3322(92)90306-r. PMID 1292648.
- ↑ "LEXOMIL - Bromazépam - Posologie, Effets secondaires, Grossesse". https://www.doctissimo.fr/medicament-LEXOMIL.htm.
- ↑ "How to Manage Common Drug Side Effects". https://www.drugs.com/article/drug-side-effects.html.
- ↑ "The subacute effect of bromazepam on psychomotor activity and subjective mood". The Journal of International Medical Research 10 (3): 140–146. 25 June 2016. doi:10.1177/030006058201000302. PMID 6124470.
- ↑ "The effect of bromazepam on psychomotor activity and subjective mood". The Journal of International Medical Research 9 (2): 89–97. 25 June 2016. doi:10.1177/030006058100900201. PMID 6112173.
- ↑ "Side effect information for Bromazepam". http://sideeffects.embl.de/drugs/2441/.
- ↑ "Side effect information for Lorazepam". http://sideeffects.embl.de/drugs/3958/.
- ↑ "Side effect information for Diazepam". http://sideeffects.embl.de/drugs/3016/.
- ↑ "Notice patient - LORAZEPAM MYLAN 1 mg, comprimé pelliculé sécable - Base de données publique des médicaments". http://base-donnees-publique.medicaments.gouv.fr/affichageDoc.php?specid=62505828&typedoc=N.
- ↑ "Dependence, misuse, and beliefs regarding use of hypnotics by elderly psychiatric patients taking zolpidem, estazolam, or flunitrazepam". Asia-Pacific Psychiatry 7 (3): 298–305. September 2015. doi:10.1111/appy.12147. PMID 25296384.
- ↑ "Prevalence of benzodiazepine abuse and dependence in psychiatric in-patients with different nosology. An assessment of hospital-based drug surveillance data". The British Journal of Psychiatry 154 (6): 839–843. June 1989. doi:10.1192/bjp.154.6.839. PMID 2574611.
- ↑ "Prevalence of prescribed benzodiazepine long-term use in the French general population according to sociodemographic and clinical factors: findings from the CONSTANCES cohort". BMC Public Health 19 (1): 566. May 2019. doi:10.1186/s12889-019-6933-8. PMID 31088561.
- ↑ "Treatment of Benzodiazepine Dependence". The New England Journal of Medicine 376 (24): 2399–2400. June 2017. doi:10.1056/NEJMc1705239. PMID 28614686. http://www.dickyricky.com/Medicine/Papers/2017_03_23%20NEJM%20Treatment%20of%20Benzodiazepine%20Dependence.pdf.
- ↑ HealthDay News (3 January 2019). "Prevalence of Benzodiazepine Use 12.6 Percent in the United States". Psychiatry Advisor. Haymarket. https://www.psychiatryadvisor.com/home/topics/anxiety/prevalence-of-benzodiazepine-use-12-6-percent-in-the-united-states/.
- ↑ "Some neurochemical correlates of "rebound" phenomenon observed during withdrawal after long-term exposure to 1, 4-benzodiazepines". Progress in Neuro-Psychopharmacology 2 (1): 43–54. 1978. doi:10.1016/0364-7722(78)90021-8. PMID 31644.
- ↑ "[A case of Lexotanil dependence. Case report on tranquilizer abuse]". Der Nervenarzt 50 (5): 326–327. May 1979. PMID 37451.
- ↑ "Rebound anxiety in anxious patients after abrupt withdrawal of benzodiazepine treatment". The American Journal of Psychiatry 141 (7): 848–852. July 1984. doi:10.1176/ajp.141.7.848. PMID 6145363.
- ↑ "New concepts in benzodiazepine therapy: rebound anxiety and new indications for the more potent benzodiazepines". Progress in Neuro-Psychopharmacology & Biological Psychiatry 7 (4–6): 669–673. 1983. doi:10.1016/0278-5846(83)90043-X. PMID 6141609.
- ↑ "[Bromazepam withdrawal delirium - a psychopharmacological contribution to clinical withdrawal syndromes (author's transl)]". Der Nervenarzt 52 (5): 293–297. May 1981. PMID 6113557.
- ↑ "De novo absence status epilepticus as a benzodiazepine withdrawal syndrome". Epilepsia 34 (2): 355–358. March 1993. doi:10.1111/j.1528-1157.1993.tb02421.x. PMID 8384109.
- ↑ "Generalized tonic-clonic seizures following withdrawal of therapeutic dose of bromazepam". Pharmacopsychiatry 32 (1): 42–43. January 1999. doi:10.1055/s-2007-979188. PMID 10071183.
- ↑ "Alterations in brain 5-hydroxytryptamine metabolism during the 'withdrawal' phase after chronic treatment with diazepam and bromazepam". British Journal of Pharmacology 60 (1): 3–9. May 1977. doi:10.1111/j.1476-5381.1977.tb16740.x. PMID 18243.
- ↑ Professor Heather Ashton (2002). "Benzodiazepines: How They Work and How to Withdraw". http://benzo.org.uk/manual/index.htm.
- ↑ "Benzodiazepine dependence--a treatment perspective and an advocacy for control". NIDA Research Monograph 131: 266–269. 1993. PMID 8105385.
- ↑ "[Drug-related toxic events in the state of São Paulo, Brazil]" (in Portuguese). Revista de Saude Publica 40 (6): 1056–1064. December 2006. doi:10.1590/s0034-89102006000700014. PMID 17173163.
- ↑ "["Alpha pattern coma" after poisoning with flunitrazepam and bromazepam. Case description]". Minerva Psichiatrica 24 (2): 69–74. 1983. PMID 6140613.
- ↑ "Difficulties in assessing brain death in a case of benzodiazepine poisoning with persistent cerebral blood flow". Human & Experimental Toxicology 23 (10): 503–505. October 2004. doi:10.1191/0960327104ht478cr. PMID 15553176.
- ↑ "How theories evolved concerning the mechanism of action of barbiturates". Epilepsia 53 (Suppl 8): 12–25. December 2012. doi:10.1111/epi.12025. PMID 23205959.
- ↑ "[Pharmacokinetics of bromazepam in 57 patients with acute drug intoxication]" (in Japanese). Chudoku Kenkyu 16 (1): 51–56. January 2003. PMID 12712542.
- ↑ "[Trends in the pharmaceutical profile of intentional drug overdoses seen in the emergency room]" (in French). Presse Médicale 34 (12): 842–846. July 2005. doi:10.1016/s0755-4982(05)84060-6. PMID 16097205.
- ↑ "Benzodiazepine poisoning in companion animals". Veterinary and Human Toxicology 37 (6): 559–562. December 1995. PMID 8588297.
- ↑ "Benzodiazepine dependence: focus on withdrawal syndrome". Annales Pharmaceutiques Françaises 67 (6): 408–413. November 2009. doi:10.1016/j.pharma.2009.07.001. PMID 19900604.
- ↑ 71.0 71.1 "Bromazepam pharmacokinetics: influence of age, gender, oral contraceptives, cimetidine, and propranolol". Clinical Pharmacology and Therapeutics 41 (5): 562–570. May 1987. doi:10.1038/clpt.1987.72. PMID 2882883.
- ↑ "[The effect of bromazepam on fitness to drive (author's transl)]" (in German). Munchener Medizinische Wochenschrift 123 (42): 1585–1588. October 1981. PMID 6118830.
- ↑ Hoffman LaRoche Pharmaceuticals (3 April 2008). "NAME OF THE MEDICINE LEXOTAN" (PDF). Australia: roche-australia.com. http://www.roche-australia.com/downloads/lexotan-pi.cfm?action=get.
- ↑ "A sudden infant death like syndrome possibly induced by a benzodiazepine in breast-feeding". European Journal of Emergency Medicine 1 (2): 86–87. June 1994. doi:10.1097/00063110-199406000-00008. PMID 9422145.
- ↑ "Clinical pharmacokinetics of fluvoxamine". Clinical Pharmacokinetics 27 (3): 175–190. September 1994. doi:10.2165/00003088-199427030-00002. PMID 7988100.
- ↑ "Progress report on the stimulant-depressant abuse liability evaluation project". NIDA Research Monograph 49: 59–62. March 1984. PMID 6148695. https://books.google.com/books?id=pA8XPwwoEMwC&pg=PA59.
- ↑ "[Presence of psychotropic drugs in the blood of drivers responsible for car accidents, and who consumed alcohol at the same time]" (in French). Sozial- und Präventivmedizin 39 (3): 143–149. May 1994. doi:10.1007/BF01299658. PMID 8048274.
- ↑ "Identification of mechanical asphyxiation in cases of attempted masking of the homicide". Forensic Science International 26 (4): 235–245. December 1984. doi:10.1016/0379-0738(84)90028-8. PMID 6519613.
- ↑ "[Chemical submission]" (in French). Presse Médicale 32 (26): 1216–1218. August 2003. PMID 14506459.
- ↑ "Chemical submission: results of 4-year French inquiry". International Journal of Legal Medicine 123 (3): 213–219. May 2009. doi:10.1007/s00414-008-0291-x. PMID 18925406.
- ↑ "Benzodiazepine Names". non-benzodiazepines.org.uk. http://www.non-benzodiazepines.org.uk/benzodiazepine-names.html.
- ↑ List of psychotropic substances under international control (PDF). International Narcotics Control Board.
- ↑ Sanal (8 January 2012). "Synthesis Of Drugs: Laboratory Synthesis Of Bromazepam". http://drugsynthesis.blogspot.com/2012/01/laboratory-synthesis-of-bromazepam.html.
External links
- Bromazepam drug information from Lexi-Comp. Includes dosage information and a comprehensive list of international brand names.
- Inchem - Bromazepam
 |
