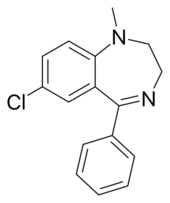
Chemistry:Medazepam
 | |
 | |
| Clinical data | |
|---|---|
| Trade names | Rudotel |
| AHFS/Drugs.com | International Drug Names |
| Routes of administration | Oral |
| ATC code | |
| Legal status | |
| Legal status |
|
| Pharmacokinetic data | |
| Bioavailability | 50–75% (Сmax = 1–2 hours) |
| Protein binding | >99% |
| Metabolism | Hepatic |
| Elimination half-life | 2 hours, 36–150 hours (terminal) |
| Excretion | Renal (63–85%), Biliary 15–37% |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEMBL | |
| Chemical and physical data | |
| Formula | C16H15ClN2 |
| Molar mass | 270.76 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| | |
Medazepam is a drug that is a benzodiazepine derivative. It possesses anxiolytic, anticonvulsant, sedative, and skeletal muscle relaxant properties. It is known by the following brand names: Azepamid, Nobrium, Tranquirax (mixed with bevonium), Rudotel, Raporan, Ansilan and Mezapam.[1] Medazepam is a long-acting benzodiazepine drug. The half-life of medazepam is 36–200 hours.[2]
Pharmacology
Medazepam acts as a prodrug to Nordazepam. Benzodiazepine drugs including medazepam increase the inhibitory processes in the cerebral cortex by allosteric modulation of the GABA receptor.[3] Benzodiazepines may also act via micromolar benzodiazepine-binding sites as Ca2+ channel blockers and significantly inhibited depolarization-sensitive calcium uptake in experiments with cell components from rat brains. This has been conjectured as a mechanism for high dose effects against seizures in a study.[4] It has major active benzodiazepine metabolites, which gives it a more prolonged therapeutic effect after administration.[5]
See also
- Benzodiazepine
- Benzodiazepine dependence
- Benzodiazepine withdrawal syndrome
- Long-term effects of benzodiazepines
References
- ↑ "Benzodiazepines". Encyclopedia of Drugs. http://www.drug-encyclopedia.eu/DW_EN/benzodiazepines.shtml.
- ↑ Ashton, Heather (April 2007). "Benzodiazepine Equivalency Table". Benzodiazepines Co-operation Not Confrontation (BCNC). http://www.bcnc.org.uk/equivalence.html.
- ↑ "Further evidence for GABA-ergic mechanisms in the action of benzodiazepines". Archives Internationales de Pharmacodynamie et de Therapie 229 (2): 313–26. October 1977. PMID 23084.
- ↑ "Micromolar-affinity benzodiazepine receptors regulate voltage-sensitive calcium channels in nerve terminal preparations". Proceedings of the National Academy of Sciences of the United States of America 81 (10): 3118–22. May 1984. doi:10.1073/pnas.81.10.3118. PMID 6328498. PMC 345232. Bibcode: 1984PNAS...81.3118T. http://www.pnas.org/cgi/reprint/81/10/3118.pdf.
- ↑ "Pharmacokinetics of benzodiazepines: metabolic pathways and plasma level profiles". Current Medical Research and Opinion 8 Suppl 4: 60–79. 1984. doi:10.1185/03007998409109545. PMID 6144464.
External links
 |

