Chemistry:Amino radical
|
| |||
| Names | |||
|---|---|---|---|
| IUPAC name
Amino radical
| |||
| Systematic IUPAC name | |||
| Other names
Amidogen; Aminyl radical; Azanyl radical
| |||
| Identifiers | |||
3D model (JSmol)
|
|||
| ChEBI | |||
| ChemSpider | |||
PubChem CID
|
|||
| |||
| |||
| Properties | |||
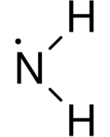
| NH2• | |||
| Molar mass | 16.0226 g mol−1 | ||
| Thermochemistry | |||
Std molar
entropy (S |
194.71 J K−1 mol−1 | ||
Std enthalpy of
formation (ΔfH⦵298) |
190.37 kJ mol−1 | ||
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |||
| Infobox references | |||
In chemistry, the amino radical, · NH
2, also known as the aminyl radical or azanyl radical, is the neutral form of the amide ion (NH−
2). Aminyl radicals are highly reactive and consequently short-lived, like most radicals; however, they form an important part of nitrogen chemistry. In sufficiently high concentration, amino radicals dimerise to form hydrazine. While NH
2 as a functional group is common in nature, forming a part of many compounds (e.g. the phenethylamines), the radical cannot be isolated in its free form.[2]
Synthesis
Reaction 1: Formation of amino radical from ammonia
Amino radicals can be produced by reacting OH radical with ammonia in irradiated aqueous solutions. This reaction is formulated as a hydrogen abstraction reaction.[3]
- [math]\ce{ NH3{} +{} ^\mathbf{\bullet}OH -> {}^\mathbf{\bullet}NH2{} + H2O }[/math]
The rate constant (k1) for this reaction was determined to be 1.0×108 M−1 s−1, while the parallel reaction of OH with NH+4 was found to be much slower. This rate was redetermined by using two-pulse radiolysis competition methods with benzoate and thiocyanate ions at pH 11.4. A value of k1 = (9 + 1)×107 M−1 s−1 was obtained from both systems. While in acidic solution, the corresponding reaction of ^ · OH with NH+
4 is too slow to be observed by pulse radiolysis.
Reaction 2: Formation of amino radical from hydroxylamine
The amino radical may also be produced by reaction of e−(aq) with hydroxylamine (NH
2OH). Several studies also utilized the redox system of TiIII
–NH
2OH for the production of amino radicals using electron paramagnetic resonance (ESR) spectroscopy and polarography.[3]
- [math]\ce{ Ti^{III}{} + NH2OH -> Ti^{IV}{} + {}^\mathbf{\bullet}NH2{} + OH- }[/math]
Reaction 3: Formation of amino radical from ammoniumyl
Reduction of hydroxylamine by e−(aq) has also been suggested to produce the amino radical in the following reaction.[3]
- [math]\ce{ ^\mathbf{\bullet}NH3+ <=> {}^\mathbf{\bullet}NH2{} + H+ }[/math]
The reactivity of the amino radical in this reaction is expected to be pH dependent and should occur in the region of pH 3–7.
Properties
Electronic states
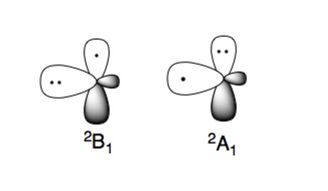
The amino radical has two characteristic electronic states:
The more stable electronic state is 2B1, where the unpaired electron is in the p-orbital perpendicular to the plane of the molecule (π type radical). The high energy electronic state, 2A1, has the two electrons in the p-orbital and the unpaired electron in the sp2 orbital (σ type radical).[4][5]
Nitrogen centered compounds, such as amines, are nucleophilic in nature. This character is also seen in amino radicals, which can be considered to be nucleophilic species.[4][5]
Spectral properties
The amino radical only exhibits a very low optical absorption in the visible region (λmax = 530 nm, εmax = 81 M−1 s−1), while its absorption in the UV (<260 nm) is similar to that of OH. Due to this, it is impractical to determine the rate of reaction of the amino radical with organic compounds by following the decay of the amino radical.
Reactivity
In general, amino radicals are highly reactive and short lived; however, this is not the case when reacted with some organic molecules. Relative reactivities of the amino radical with several organic compounds have been reported, but the absolute rate constants for such reactions remain unknown. In reaction 1, it was hypothesized that the amino radical might possibly react with NH3 more rapidly than OH and might oxidize NH+4 to produce the amino radical in acid solutions, given that radicals are stronger oxidants than OH. In order to test this, sulfate and phosphate radical anions were used. The sulfate and phosphate radical anions were found to react more slowly with NH3 than does the amino radical and they react with ammonia by hydrogen abstraction and not by electron transfer oxidation.[3]
When the amino radical is reacted with benzoate ions, the rate constant is very low and only a weak absorption in the UV spectra is observed, indicating that amino radicals do not react with benzene rapidly. Phenol, on the other hand, was found to react more rapidly with the amino radical. In experiments at pH 11.3 and 12, using 1.5 M NH3 and varying concentrations of phenol between 4 and 10 mM, the formation of the phenoxyl radical absorption was observed with a rate constant of (3 + 0.4)×106 M−1 s−1. This reaction can produce phenoxyl radicals via two possible mechanisms:[3]
- Addition to the ring followed by elimination of NH3, or
- Oxidation by direct electron transfer

While the amino radical is known to be weakly reactive, the recombination process of two amino radicals to form hydrazine appears to be one of the fastest. As a result, it often competes with other NH2 reactions.
- NH2 + NH2 → N2H4
At low pressures, this reaction is the fastest and therefore the principal mode of NH2 disappearance.[6]
See also
References
- ↑ 1.0 1.1 "aminyl (CHEBI:29318)". Chemical Entities of Biological Interest (ChEBI). UK: European Bioinformatics Institute. IUPAC Names. https://www.ebi.ac.uk/chebi/searchId.do?chebiId=29318.
- ↑ die.net. "Amidogen". http://dictionary.die.net/amidogen.
- ↑ 3.0 3.1 3.2 3.3 3.4 3.5 Neta, P.; Maruthamuthu, P.; Carton, P. M.; Fessenden, R. W. (1978). "Formation and reactivity of the amino radical". The Journal of Physical Chemistry 82 (17): 1875–1878. doi:10.1021/j100506a004. ISSN 0022-3654.
- ↑ 4.0 4.1 "Amino Radical". NIST Chemistry WebBook. National Institute of Science and Technology. 2017. https://webbook.nist.gov/cgi/cbook.cgi?ID=C13770406&Mask=6FF.
- ↑ 5.0 5.1 Koenig, T.; Hoobler, J. A.; Klopfenstein, C. E.; Hedden, G.; Sunderman, F.; Russell, B. R. (1974). "Electronic configurations of amido radicals". Journal of the American Chemical Society 96 (14): 4573–4577. doi:10.1021/ja00821a036. ISSN 0002-7863.
- ↑ Khe, P. V.; Soulignac, J. C.; Lesclaux, R. (1977). "Pressure and temperature dependence of amino radical recombination rate constant". The Journal of Physical Chemistry 81 (3): 210–214. doi:10.1021/j100518a006.
Further reading
- Davies, P (2008). "Detection of the amino radical NH2 by laser magnetic resonance spectroscopy". The Journal of Chemical Physics 62 (9): 3739–3742. doi:10.1063/1.430970.
- Buttner, T (2005). "A stable aminyl radical metal complex". Science 307 (5707): 235–8. doi:10.1126/science.1106070. PMID 15653498. Bibcode: 2005Sci...307..235B.
- John, Seely (1977). "Temperature and Pressure Dependence of the Rate Constant for the HO2 + NO Reaction". The Journal of Physical Chemistry 81 (10): 210–214. doi:10.1021/jp952553f.
- Koenig, Hoobler (1974). "Electronic configurations of amino radicals". Journal of the American Chemical Society 96 (14): 4573–4577. doi:10.1021/ja00821a036.
 |