Chemistry:Ciclopirox
 | |
| Clinical data | |
|---|---|
| Trade names | Many[1] |
| Other names | Loprox, CPX |
| AHFS/Drugs.com | Micromedex Detailed Consumer Information |
| MedlinePlus | a604021 |
| Pregnancy category |
|
| Routes of administration | Topical |
| ATC code | |
| Legal status | |
| Legal status | |
| Pharmacokinetic data | |
| Bioavailability | <5% with prolonged use |
| Protein binding | 94 to 97% |
| Elimination half-life | 1.7 hours |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEBI | |
| ChEMBL | |
| Chemical and physical data | |
| Formula | C12H17NO2 |
| Molar mass | 207.273 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| (verify) | |
Ciclopirox is a synthetic antifungal agent for topical dermatologic treatment of superficial mycoses. It is most useful against tinea versicolor. It is often used clinically as ciclopirox olamine, the olamine salt of ciclopirox.
In 2021, it was the 271st most commonly prescribed medication in the United States, with more than 900,000 prescriptions.[2][3]
Medical uses
Ciclopirox is indicated for the treatment of tinea pedis and tinea corporis due to Trichophyton rubrum, Trichophyton mentagrophytes and Epidermophyton floccosum, as well as seborrheic dermatitis. It is not to be used in the eyes or vagina, and nursing women should consult their doctors before use, since it is not known whether ciclopirox passes into human milk. A burning sensation may be felt when first applying ciclopirox on the skin.[citation needed]
Nail infections
In addition to other formulations, ciclopirox is used in lacquers for topical treatment of onychomycosis (fungal infections of the nails). A meta-analysis of the six trials of nail infections available in 2009 concluded that they provided evidence that topical ciclopirox had poor cure rates, and that amorolfine might be substantially more effective, but more research was required. "Combining data from 2 trials of ciclopiroxolamine versus placebo found treatments failure rates of 61% and 64% for ciclopiroxolamine. These outcomes followed long treatment times (48 weeks) and this makes ciclopiroxolamine a poor choice for nail infections. Better results were observed with the use of amorolfine lacquer; 6% treatment failure rates were found after 1 month of treatment but these data were collected on a very small sample of people and these high rates of success might be unreliable."[4]
Efinaconazole, an azole antifungal, led to cure rates two or three times better than the next-best topical treatment, ciclopirox.[5]
Pharmacology
Pharmacodynamics
In contrast to the azoles and other antimycotic drugs, the mechanism of action of ciclopirox is poorly understood.[6] However, loss of function of certain catalase and peroxidase enzymes has been implicated as the mechanism of action, as well as various other components of cellular metabolism. In a study conducted to further elucidate ciclopirox's mechanism, several Saccharomyces cerevisiae mutants were screened and tested. Results from interpretation of the effects of both the drug treatment and mutation suggested that ciclopirox may exert its effect by disrupting DNA repair, cell division signals and structures (mitotic spindles) as well as some elements of intracellular transport.[7]
Chemistry
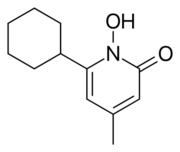
Ciclopirox is a N-hydroxypyridone. Structurally, ciclopirox is the N-oxide of a 2-hydroxypyridine derivative and therefore can be termed a hydroxypyridine antifungal agent. Additionally, the structure as drawn above is the lactam tautomer and indicates the molecule being an N-hydroxy-2-pyridone. Hence the classification of ciclopirox as a 2-pyridone antifungal agent.[citation needed]
Society and culture
Brand names
It is sold under many brand names worldwide.[1]
Research
In 2007 it was investigated as an alternative treatment to ketoconazole for seborrhoeic dermatitis as it suppresses growth of the yeast Malassezia furfur. Initial results showed similar efficacy to ketoconazole with a relative increase in subjective symptom relief due to its inherent anti-inflammatory properties.[8]
References
- ↑ 1.0 1.1 Drugs.com International brand names for ciclopirox Page accessed January 201, 2016
- ↑ "The Top 300 of 2021". https://clincalc.com/DrugStats/Top300Drugs.aspx.
- ↑ "Ciclopirox - Drug Usage Statistics". https://clincalc.com/DrugStats/Drugs/Ciclopirox.
- ↑ "Topical treatments for fungal infections of the skin and nails of the foot". The Cochrane Database of Systematic Reviews 2007 (3): CD001434. 2007. doi:10.1002/14651858.CD001434.pub2. PMID 17636672.
- ↑ "A Closer Look At A New Topical Option For Onychomycosis". http://www.podiatrytoday.com/blogged/closer-look-new-topical-option-onychomycosis.
- ↑ "Ciclopirox Olamine Treatment Affects the Expression Pattern of Candida albicans Genes Encoding Virulence Factors, Iron Metabolism Proteins, and Drug Resistance Factors". Antimicrobial Agents and Chemotherapy 47 (6): 1805–1817. June 2003. doi:10.1128/AAC.47.6.1805-1817.2003. PMID 12760852.
- ↑ "The possible mechanism of action of ciclopirox olamine in the yeast Saccharomyces cerevisiae". Mol. Cells 15 (1): 55–61. 2003. doi:10.1016/S1016-8478(23)13707-1. PMID 12661761. http://www.molcells.org/home/journal/article_read.asp?volume=15&number=1&startpage=55. Retrieved 2008-08-15.
- ↑ "Clinical efficacies of shampoos containing ciclopirox olamine (1.5%) and ketoconazole (2.0%) in the treatment of seborrhoeic dermatitis". J Dermatolog Treat 18 (2): 88–96. 2007. doi:10.1080/16537150601092944. PMID 17520465.
 |

