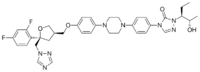
Chemistry:Posaconazole
 | |
 | |
| Clinical data | |
|---|---|
| Trade names | Noxafil, Posanol, others |
| AHFS/Drugs.com | Monograph |
| MedlinePlus | a607036 |
| License data |
|
| Pregnancy category |
|
| Routes of administration | By mouth, intravenous |
| ATC code | |
| Legal status | |
| Legal status | |
| Pharmacokinetic data | |
| Bioavailability | Low (8 to 47% Oral) |
| Protein binding | 98 to 99% |
| Metabolism | Liver (glucuronidation) |
| Elimination half-life | 16 to 31 hours |
| Excretion | Fecal (71–77%) and Kidney (13–14%) |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEBI | |
| ChEMBL | |
| Chemical and physical data | |
| Formula | C37H42F2N8O4 |
| Molar mass | 700.792 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| | |
Posaconazole, sold under the brand name Noxafil among others, is a triazole antifungal medication.[7][8]
It was approved for medical use in the United States in September 2006,[5][9] and is available as a generic medication.[10][11]
Medical uses
Posaconazole is used to treat invasive Aspergillus and Candida infections.[5] It is also used for the treatment of oropharyngeal candidiasis (OPC), including OPC refractory to itraconazole and/or fluconazole therapy.[5]
It is also used to treat invasive infections by Candida, Mucor, and Aspergillus species in severely immunocompromised patients.[12][13]
Clinical evidence for its utility in treatment of invasive disease caused by Fusarium species (fusariosis) is limited.[14]
It appears to be helpful in a mouse model of naegleriasis.[15]
Pharmacology
Pharmacodynamics
Posaconazole works by disrupting the close packing of acyl chains of phospholipids, impairing the functions of certain membrane-bound enzyme systems such as ATPase and enzymes of the electron transport system, thus inhibiting growth of the fungi. It does this by blocking the synthesis of ergosterol by inhibiting of the enzyme lanosterol 14α-demethylase and accumulation of methylated sterol precursors. Posaconazole is significantly more potent at inhibiting 14-alpha demethylase than itraconazole.[5][16][17]
Microbiology
Posaconazole is active against the following microorganisms:[16][18]
- Candida spp.
- Aspergillus spp.
- Zygomycetes spp.
Pharmacokinetics
Posaconazole is absorbed within three to five hours. It is predominantly eliminated through the liver, and has a half-life of about 35 hours. Oral administration of posaconazole taken with a high-fat meal exceeds 90% bioavailability and increases the concentration by four times compared to fasting state.[5][18]
References
- ↑ "Posaconazole (Noxafil) Use During Pregnancy". 23 April 2019. https://www.drugs.com/pregnancy/posaconazole.html.
- ↑ "Posaconazole suspension ARX/Posaconazole TIH/APX-Posaconazole (Arrow Pharma Pty Ltd)". 16 February 2023. https://www.tga.gov.au/resources/prescription-medicines-registrations/posaconazole-suspension-arxposaconazole-tihapx-posaconazole-arrow-pharma-pty-ltd.
- ↑ "Posanol Product information". 25 April 2012. https://health-products.canada.ca/dpd-bdpp/info.do?lang=en&code=77877.
- ↑ "Noxafil 100 mg Gastro-resistant Tablets - Summary of Product Characteristics (SmPC)". 10 January 2022. https://www.medicines.org.uk/emc/product/5388/smpc.
- ↑ 5.0 5.1 5.2 5.3 5.4 5.5 "Noxafil- posaconazole suspension Noxafil- posaconazole tablet, coated Noxafil- posaconazole solution". 20 March 2020. https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=b073b082-7b57-4423-8c06-4fd4263d6f84.
- ↑ "Noxafil EPAR". 17 September 2018. https://www.ema.europa.eu/en/medicines/human/EPAR/noxafil.
- ↑ "Posaconazole: an extended-spectrum triazole antifungal agent". Clinical Therapeutics 29 (9): 1862–86. September 2007. doi:10.1016/j.clinthera.2007.09.015. PMID 18035188.
- ↑ "Posaconazole: an oral triazole with an extended spectrum of activity". The Annals of Pharmacotherapy 42 (10): 1429–38. October 2008. doi:10.1345/aph.1L005. PMID 18713852. http://www.theannals.com/cgi/pmidlookup?view=long&pmid=18713852. Retrieved 11 December 2008.[|permanent dead link|dead link}}]
- ↑ "Drug Approval Package: Noxafil (Posaconazole) NDA #022003". 9 November 2006. https://www.accessdata.fda.gov/drugsatfda_docs/nda/2006/022003s000_NoxafilTOC.cfm.
- ↑ "Posaconazole: FDA-Approved Drugs". https://www.accessdata.fda.gov/scripts/cder/daf/index.cfm?event=overview.process&ApplNo=212411.
- ↑ "First Generic Drug Approvals". 17 October 2022. https://www.fda.gov/drugs/drug-and-biologic-approval-and-ind-activity-reports/first-generic-drug-approvals.
- ↑ "Changes in susceptibility to posaconazole in clinical isolates of Candida albicans". The Journal of Antimicrobial Chemotherapy 53 (1): 74–80. January 2004. doi:10.1093/jac/dkh027. PMID 14657086.
- ↑ "Treatment of invasive aspergillosis with posaconazole in patients who are refractory to or intolerant of conventional therapy: an externally controlled trial". Clinical Infectious Diseases 44 (1): 2–12. January 2007. doi:10.1086/508774. PMID 17143808. (Subscription content?)
- ↑ "Posaconazole as salvage treatment for invasive fusariosis in patients with underlying hematologic malignancy and other conditions". Clinical Infectious Diseases 42 (10): 1398–403. May 2006. doi:10.1086/503425. PMID 16619151.
- ↑ "Phenotypic Screens Reveal Posaconazole as a Rapidly Acting Amebicidal Combination Partner for Treatment of Primary Amoebic Meningoencephalitis". The Journal of Infectious Diseases 219 (7): 1095–1103. March 2019. doi:10.1093/infdis/jiy622. PMID 30358879.
- ↑ 16.0 16.1 Brunton L, Lazo J, Parker K. Goodman and Gilman's The Pharmacological Basis of Therapeutics. 11th ed. San Francisco: McGraw-Hill; 2006. ISBN 978-0-07-142280-2
- ↑ "Clinical Pharmacology Posaconazole". http://www.clinicalpharmacology-ip.com/Forms/Monograph/monograph.aspx?cpnum=2811&sec=monmech.
- ↑ 18.0 18.1 "Pharmacology of azoles". UpToDate. Waltham, MA: UpToDate. October 2017. http://www.utdol.com/online/content/topic.do?topicKey=antibiot/9969&selectedTitle=4%7E38&source=_result#H9. Retrieved 18 February 2010.
External links
 |
