Chemistry:Telluric acid
| |
| |
| Names | |
|---|---|
| IUPAC name
Hexahydroxidotellurium
| |
Other names
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChEBI | |
| ChemSpider | |
PubChem CID
|
|
| UNII | |
| |
| |
| Properties | |
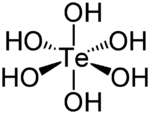
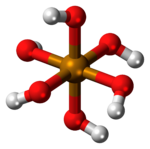
| Te(OH) 6 | |
| Molar mass | 229.64 g·mol−1 |
| Appearance | White monoclinic crystals |
| Density | 3.07 g/cm3 |
| Melting point | 136 °C (277 °F; 409 K) |
| 50.1 g/(100 ml) at 30 °C[1] | |
| Acidity (pKa) | 7.68, 11.0 at 18 °C[1] |
| Conjugate base | Tellurate |
| Structure | |
| octahedral | |
| 0 D | |
| Hazards | |
| Main hazards | corrosive |
| Related compounds | |
Other anions
|
Hydrotelluric acid Tellurous acid Hydrogen telluride |
Related compounds
|
Teflic acid Sulfuric acid Selenic acid |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Telluric acid, or more accurately orthotelluric acid, is a chemical compound with the formula Te(OH)
6, often written as H
6TeO
6. It is a white crystalline solid made up of octahedral Te(OH)
6 molecules which persist in aqueous solution.[2] In the solid state, there are two forms, rhombohedral and monoclinic, and both contain octahedral Te(OH)
6 molecules,[3] containing one hexavalent tellurium (Te) atom in the +6 oxidation state, attached to six hydroxyl (–OH) groups, thus, it can be called tellurium(VI) hydroxide.
Telluric acid is a weak acid which is dibasic, forming tellurate salts with strong bases and hydrogen tellurate salts with weaker bases or upon hydrolysis of tellurates in water.[3][4] It is used as tellurium-source in the synthesis of oxidation catalysts.
Preparation
Telluric acid is formed by the oxidation of tellurium or tellurium dioxide with a powerful oxidising agent such as hydrogen peroxide, chromium trioxide or sodium peroxide.[3]
- TeO
2 + H
2O
2 + 2 H
2O → Te(OH)
6
Crystallization of telluric acid solutions below 10 °C gives telluric acid tetrahydrate Te(OH)
6 · 4H2O.[2]
It is an oxidising agent, as shown by the electrode potential for the reaction below, although it is kinetically slow in its oxidations.[3]
- Te(OH)
6 + 2 H+
+ 2 e−
⇌ TeO
2 + 4 H
2O, Eo= +1.02 V
Chlorine, by comparison, is +1.36 V and selenous acid is +0.74 V in oxidizing conditions.
Properties and reactions
The anhydrous acid is stable in air at 100 °C but above this it dehydrates to form polymetatelluric acid, a white hygroscopic powder (approximate composition (H
2TeO
4)
10), and allotelluric acid, an acid syrup of unknown structure (approximate composition 3 · H
2TeO
4 · 4H2O).[5][2]
Typical salts of the acid contains the anions [Te(O)(OH)
5]−
and [Te(O)
2(OH)
4]2−. The presence of the tellurate ion TeO2−
4 has been confirmed in the solid state structure of Rb
6[TeO
5][TeO
4].[6]
Strong heating at over 300 °C produces the α crystalline modification of tellurium trioxide, α-TeO
3.
[4] Reaction with diazomethane gives the hexamethyl ester, Te(OCH
3)
6.[2]
Telluric acid and its salts mostly contain hexacoordinate tellurium.[3] This is true even for salts such as magnesium tellurate, MgTeO
4, which is isostructural with magnesium molybdate and contains TeO
6 octahedra.[3]
Other forms of telluric acid
Metatelluric acid, H
2TeO
4, the tellurium analogue of sulfuric acid, H
2SO
4, is unknown. Allotelluric acid of approximate composition 3 · H
2TeO
4 · 4H2O, is not well characterised and may be a mixture of Te(OH)
6 and (H
2TeO
4)
n.[2]
Other tellurium acids
Tellurous acid H
2TeO
3, containing tellurium in its +4 oxidation state, is known but not well characterised.
Hydrogen telluride is an unstable gas that forms hydrotelluric acid upon addition to water.
References
- ↑ 1.0 1.1 Lide, David R. (1998), Handbook of Chemistry and Physics (87 ed.), Boca Raton, Florida: CRC Press, ISBN 0-8493-0594-2
- ↑ 2.0 2.1 2.2 2.3 2.4 Greenwood, Norman N.; Earnshaw, Alan (1997). Chemistry of the Elements (2nd ed.). Butterworth-Heinemann. ISBN 978-0-08-037941-8.
- ↑ 3.0 3.1 3.2 3.3 3.4 3.5 Cotton, F. Albert; Wilkinson, Geoffrey; Murillo, Carlos A.; Bochmann, Manfred (1999), Advanced Inorganic Chemistry (6th ed.), New York: Wiley-Interscience, ISBN 0-471-19957-5
- ↑ 4.0 4.1 Holleman, A. F.; Wiberg, E. "Inorganic Chemistry" Academic Press: San Diego, 2001. ISBN:0-12-352651-5.
- ↑ Loub, J.; Haase, W.; Mergehenn, R. (1979). "Structure of an adduct of orthotelluric acid and urea". Acta Crystallographica Section B: Structural Crystallography and Crystal Chemistry 35 (12): 3039–3041. doi:10.1107/S0567740879011286.
- ↑ Catherine E. Housecroft; Alan G. Sharpe (2008). "Chapter 16: The group 16 elements". Inorganic Chemistry, 3rd Edition. Pearson. p. 526. ISBN 978-0-13-175553-6.
 |

