Chemistry:Picolinic acid
| |
| |
| Names | |
|---|---|
| Preferred IUPAC name
Pyridine-2-carboxylic acid | |
| Other names
Picolinic acid
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
PubChem CID
|
|
| UNII | |
| |
| |
| Properties | |
| C6H5NO2 | |
| Molar mass | 123.111 g·mol−1 |
| Appearance | White to tan crystalline solid |
| Melting point | 136 to 138 °C (277 to 280 °F; 409 to 411 K) |
| Slightly soluble (0.41%) in water[1] | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Picolinic acid is an organic compound with the formula C5H4NCOOH). It is a derivative of pyridine with a carboxylic acid (COOH) substituent at the 2-position. It is an isomer of nicotinic acid and isonicotinic acid, which have the carboxyl side chain at the 3- and 4-positions, respectively. It is a white solid that is soluble in water.
In synthetic organic chemistry, has been used as a substrate in the Mitsunobu reaction and in the Hammick reaction.[2]
Coordination chemistry
Picolinic acid is a bidentate chelating agent of elements such as chromium, zinc, manganese, copper, iron, and molybdenum in the human body.[3]:72 Many of its complexes are charge-neutral and thus lipophilic. After its role in absorption was discovered, zinc picolinate dietary supplements became popular as they were shown to be an effective means of introducing zinc into the body.[3]
Production
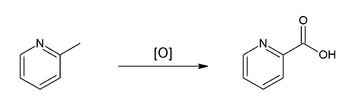
Picolinic acid is formed from 2-methylpyridine by oxidation, e.g. by means of potassium permanganate (KMnO4).[4][5]
Biosynthesis
Picolinic acid is a catabolite of the amino acid tryptophan through the kynurenine pathway.[3][6] Its function is unclear, but it has been implicated in a variety of neuroprotective, immunological, and anti-proliferative effects. In addition, it is suggested to assist in the absorption of zinc(II) ions and other divalent or trivalent ions through the small intestine.[7]
Picolinates
Salts of picolinic acid (picolinates) include:
See also
References
- ↑ Lide, DR. CRC Handbook of Chemistry and Physics, Internet Version 2005, http://hbcpnetbase.com, CRC Press, Boca Raton, Florida, 2005.
- ↑ Fuchs, Philip L. (2013-07-29). "Picolinic acid". Catalytic Oxidation Reagents. Wiley Inc.. p. 495ff. ISBN 9781118704844. OCLC 954583821. https://books.google.com/books?id=Cbc2AAAAQBAJ&q=picolinic%20acid.
- ↑ 3.0 3.1 3.2 Grant, RS; Coggan, SE; Smythe, GA (2009). "The physiological action of picolinic Acid in the human brain.". International Journal of Tryptophan Research 2: 71–9. doi:10.4137/ijtr.s2469. PMID 22084583.
- ↑ Shimizu, Shinkichi; Watanabe, Nanao; Kataoka, Toshiaki; Shoji, Takayuki; Abe, Nobuyuki; Morishita, Sinji; Ichimura, Hisao (2007). "Ullmann's Encyclopedia of Industrial Chemistry". Ullmann's Encyclopedia of Industrial Chemistry. Weinheim: Wiley-VCH. doi:10.1002/14356007.a22_399.
- ↑ Harold Hart (Autor), Leslie E. Craine (Autor), David J. Hart (Autor), Christopher M. Hadad (Autor); Nicole Kindler (Übersetzer): Organische Chemie, 3. Auflage, Wiley-VCH, Weinheim 2007, ISBN:978-3-527-31801-8, S. 494.
- ↑ Tan, L. (December 2012). "The kynurenine pathway in neurodegenerative diseases: mechanistic and therapeutic considerations". J Neurol Sci 323 (1–2): 1–8. doi:10.1016/j.jns.2012.08.005. PMID 22939820.
- ↑ Evans, Gary (1982). "The Role of Picolinic Acid in Metal Metabolism". Life Chemistry Reports (Harwood Academic Publishers) 1: 57–67. http://naldc.nal.usda.gov/download/46436/PDF. Retrieved 20 March 2015.
 |