Chemistry:Tribromoethanol
 | |
| Clinical data | |
|---|---|
| Trade names | Avertin |
| Other names | Tribromoethyl alcohol |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| Chemical and physical data | |
| Formula | C2H3Br3O |
| Molar mass | 282.757 g·mol−1 |
| 3D model (JSmol) | |
| Melting point | 73–79 °C (163–174 °F) [1][2] |
| Boiling point | 92–93 °C (198–199 °F) at 10 mmHg[1] |
| |
| |
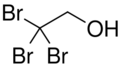
2,2,2-Tribromoethanol, often called just tribromoethanol, is a chemical compound with formula Br
3C–CH
2OH. Its molecule can be described as that of ethanol, with the three hydrogen atoms in position 2 (on the methyl group) replaced by bromine. It is a white crystalline solid, soluble in water and other solvents, that absorbs strongly in the UV below 290 nm.[2]
Tribromoethanol is used in medicine and biology as an anesthetic, and has been available commercially for that purpose by the trade name Avertin. It was formerly used on humans[3] and is still often used on laboratory animals,[4] and to capture wild birds.[5] It is also used in plastics industry as a polymerization initiator.[6][7]
Uses
Animal anesthetic
Tribromoethanol is often used to anesthetize laboratory animals, particularly rodents, before surgery.[4] As a solution in tert-amyl alcohol, it has the brand name Avertin.[8] The tert-amyl alcohol acts as a weak hypnotic, in addition to improving the solubility of the tribromoethanol. Administered intravenously, tribromoethanol (Avertin) causes rapid and deep anesthesia followed by rapid and full postoperative recovery in small mammals.[9] Recently its safety for this purpose has been questioned.[10]
Wildlife capture
Tribromoethanol has also been long used as spiked grain bait to capture wild turkeys for research and wildlife management purposes.[5] However, the birds learn to avoid it, for over a year, after a single exposure, and will drive other flock members away from the bait when they detect it.[11]
Human anesthetic
In the first half of the 20th century, Avertin was also used in humans as a general anesthetic or basal narcotic to induce unconsciousness prior to the administration of other anesthetic agents. It was administered rectally as a retention enema or by intravenous injection. Its rectal use was particularly favored for pediatrics, head or neck surgery, or in mentally unstable or anxious patients.[3] Electrophysiology studies showed that tribromoethanol acts as a positive allosteric modulator of the inhibitory GABAA and glycine receptors, a mechanism similar to that seen with the related compound 2,2,2-trichloroethanol.[12] Bromal hydrate (2,2,2-tribromoethanol-1,1-diol), a compound also recognized to produce general anesthesia in animals, is metabolized to tribromoethanol.[13]
Polymerization initiator
Tribromoethanol can be used as a functional polymerization initiator for the introduction of α-hydroxyl groups in poly(methyl methacrylate) (PMMA) and poly(n-butyl acrylate) (PBAK).[6][14][7]
Chemistry
Photolysis
Tribromoethanol should be kept refrigerated and in the dark, to prevent decomposition by light into hydrobromic acid and possibly 2,2-dibromoacetaldehyde or glycolic acid.[15][2]
See also
- Trichloroethanol
- Trifluoroethanol
References
- ↑ 1.0 1.1 "2,2,2-Tribromoethanol". Sigma-Aldrich. http://www.sigmaaldrich.com/catalog/product/aldrich/t48402?lang=en.
- ↑ 2.0 2.1 2.2 "Radiolysis of aqueous 2,2,2-tribromoethanol solutions.". Canadian Journal of Chemistry 48 (16): 2542–2548. August 1970. doi:10.1139/v70-430.
- ↑ 3.0 3.1 "Tribromethyl alcohol (avertin, bromethol), 1928-1945". Proceedings of the Royal Society of Medicine 39 (2): 71–76. December 1945. doi:10.1177/003591574503900203. PMID 21010258.
- ↑ 4.0 4.1 "Tribromoethanol (Avertin)". Cold Spring Harbor Protocols (Cold Spring Harbor Laboratory) 2006: pdb.rec701. 2006. doi:10.1101/pdb.rec701. http://cshprotocols.cshlp.org/content/2006/1/pdb.rec701.full?text_only=true.
- ↑ 5.0 5.1 "Capturing wild turkeys with tribromoethanol.". The Journal of Wildlife Management 39 (3): 630–634. July 1975. doi:10.2307/3800410.
- ↑ 6.0 6.1 "Controlled radical polymerization of (meth) acrylates by ATRP with NiBr2 (PPh3) 2 as catalyst.". Macromolecules 32 (1): 27–35. January 1999. doi:10.1021/ma980995u.
- ↑ 7.0 7.1 "Styrene ATRP using the new initiator 2,2,2‐tribromoethanol: Experimental and simulation approach.". Polymer Engineering & Science 55 (10): 2270–2276. October 2015. doi:10.1002/pen.24113.
- ↑ "Guidelines for the Use of Tribromoethanol/Avertin Anesthesia". National Cancer Institute. https://ncifrederick.cancer.gov/lasp/acuc/frederick/Media/Documents/ACUC32.pdf.
- ↑ "Tribromoethanol (Avertin) as an anaesthetic in mice". Laboratory Animals 33 (2): 192–193. April 1999. doi:10.1258/002367799780578417. PMID 10780824.
- ↑ Robert E. Meyer and Richard E. Fish (2005) "A review of tribromoethanol anesthesia for production of genetically engineered mice and rats". Lab Animal, volume 34, pages 47–52. "A review of tribromoethanol anesthesia for production of genetically engineered mice and rats". Lab Animal 34 (10): 47–52. November 2005. doi:10.1038/laban1105-47. PMID 16261153.
- ↑ "Feasibility of Using Tribromoethanol to Recapture Wild Turkeys.". Wildlife Society Bulletin 22 (3): 496–500. October 1994.
- ↑ "The actions of ether, alcohol and alkane general anaesthetics on GABAA and glycine receptors and the effects of TM2 and TM3 mutations". British Journal of Pharmacology 129 (4): 731–743. February 2000. doi:10.1038/sj.bjp.0703087. PMID 10683198.
- ↑ "Trichlorethanol, tribromethanol, chloral hydrate and bromal hydrate.". Journal of Pharmacology and Experimental Therapeutics 63 (4): 453–65. August 1938. http://jpet.aspetjournals.org/content/63/4/453.short.
- ↑ "Synthesis and characterization of polystyrene-block-polylactide by combination of ATRP and ROP using tribromoethanol as initiator: Precursors to ordered nanoporous materials.". 236th ACS National Meeting 49 (2): 302–303. 2008. https://hal.archives-ouvertes.fr/hal-00356594.
- ↑ "Protective effect of oestrogens against the toxic decomposition products of tribromoethanol". Nature 208 (5015): 1098–1099. December 1965. doi:10.1038/2081098a0. PMID 5331549.
 |
