Chemistry:Laudanosine
| |
| |
| Names | |
|---|---|
| Preferred IUPAC name
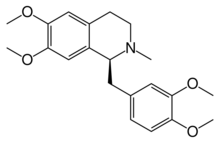
(1S)-1-[(3,4-Dimethoxyphenyl)methyl]-6,7-dimethoxy-2-methyl-1,2,3,4-tetrahydroisoquinoline | |
| Other names
N-Methyl-1,2,3,4-tetrahydropapaverine
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChemSpider | |
| EC Number |
|
PubChem CID
|
|
| UNII | |
| |
| |
| Properties | |
| C21H27NO4 | |
| Molar mass | 357.450 g·mol−1 |
| Melting point | 89 °C (192 °F; 362 K) |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Laudanosine or N-methyltetrahydropapaverine is a recognized metabolite[1] of atracurium and cisatracurium. Laudanosine decreases the seizure threshold, and thus it can induce seizures if present at sufficient threshold concentrations; however such concentrations are unlikely to be produced consequent to chemodegradable metabolism of clinically administered doses of cisatracurium or atracurium.
Laudanosine also occurs naturally in minute amounts (0.1%) in opium, from which it was first isolated in 1871.[2] Partial dehydrogenation of laudanosine will lead to papaverine, the alkaloid found in the opium poppy plant (Papaver somniferum).
Laudanosine is a benzyltetrahydroisoquinoline alkaloid. It has been shown to interact with GABA receptors, glycine receptors, opioid receptors, and nicotinic acetylcholine receptors,[1][3][4] but not benzodiazepine or muscarinic receptors, which are also involved in epilepsy and other types of seizures.[5]
References
- ↑ 1.0 1.1 "Laudanosine, an atracurium and cisatracurium metabolite". Eur J Anaesthesiol 19 (7): 466–73. July 2002. doi:10.1017/s0265021502000777. PMID 12113608.
- ↑ Burger A (2005). "The Benzylisoquinoline Alkaloids". The Alkaloids: Chemistry and Physiology. 4. New York: Academic Press. pp. 48. ISBN 0-12-469504-3. https://books.google.com/books?id=KxTKLlac60wC&q=laudanosine&pg=PA55. Retrieved September 18, 2008 through Google Book Search.
- ↑ "Interactions between laudanosine, GABA, and opioid subtype receptors: implication for laudanosine seizure activity". Brain Res 646 (2): 235–241. May 1994. doi:10.1016/0006-8993(94)90084-1. PMID 8069669.
- ↑ "Evaluation of benzyltetrahydroisoquinolines as ligands for neuronal nicotinic acetylcholine receptors". Br J Pharmacol 146 (1): 15–24. Sep 2005. doi:10.1038/sj.bjp.0706307. PMID 15980871.
- ↑ "Laudanosine does not displace receptor-specific ligands from the benzodiazepinergic or muscarinic receptors". Anesthesiology 70 (1): 109–111. Jan 1989. doi:10.1097/00000542-198901000-00020. PMID 2536252.
 |


