Chemistry:Iron(II) nitrate
| |

| |
| Names | |
|---|---|
Other names
| |
| Identifiers | |
| |
3D model (JSmol)
|
|
| ChemSpider | |
| EC Number |
|
PubChem CID
|
|
| UNII |
|
| |
| |
| Properties | |
| Fe(NO3)2 | |
| Molar mass | 179.86 g/mol |
| Appearance | Green crystals (hexahydrate) |
| Melting point | 60 °C (140 °F; 333 K)[2] (hexahydrate) |
| Boiling point | 61 °C (142 °F; 334 K)[1] (decomposes) |
| 87.525 g/100 mL | |
| Structure | |
| Orthorhombic[3] | |
| Thermochemistry | |
Std enthalpy of
formation (ΔfH⦵298) |
-497.9 kJ/mol[1] |
| Hazards | |
| Lethal dose or concentration (LD, LC): | |
LD50 (median dose)
|
428 mg/kg (subcutaneous, rabbit)[4] |
| Related compounds | |
Other anions
|
Iron(II) phosphate |
Other cations
|
Manganese(II) nitrate Cobalt(II) nitrate |
Related compounds
|
Iron(III) nitrate |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
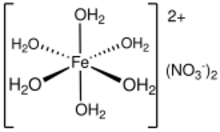
Iron(II) nitrate is the nitrate salt of iron(II). It is commonly encountered as the green hexahydrate, Fe(NO3)2·6H2O, which is a metal aquo complex, however it is not commercially available unlike iron(III) nitrate due to its instability to air. The salt is soluble in water serves as a ready source of ferrous ions.
Structure
No structure of any salt Fe(NO3)2·xH2O has been determined by X-ray crystallography. Nonetheless, the nature of the aquo complex [Fe(H2O)6]2+ is well known and relatively insensitive to the anion. The Fe-O distances are longer for [Fe(H2O)6]2+ (2.13 Å) than for the ferric analogue [Fe(H2O)6]3+ (1.99 Å).[5] Both [Fe(H2O)6]n+ complexes are high spin, which results in pale colors, paramagnetism, and weak Fe-O bonds.
Production
Iron(II) nitrate can be produced in multiple ways, such as the reaction of iron metal with cold dilute nitric acid:
- 3 Fe + 8 HNO3 + 12 H2O → 3 Fe(NO3)2(H2O)6 + 2 NO
If this reaction is conducted below -10 °C, nonahydrate is produced. It readily releases water to give the hexahydrate.[1]
The above reaction can also co-produce ferric nitrate. Reacting iron(II) sulfate and lead nitrate under dilute ethanol and then evaporating the solution leads to the formation of the green crystals of the hexahydrate. A solution of iron(II) nitrate is produced by the ion-exchange reaction of iron(II) sulfate and barium nitrate, producing a concentration of up to 1.5 M due to the limited solubility of barium nitrate.[1][6][7]
The solution of the iron(II) nitrate-hydrazine complex is produced by the reaction of hydrazine nitrate and ferric nitrate at 40 °C with copper(II) nitrate as a catalyst:[8]
- 4 Fe(NO3)3 + N2H5NO3 → 4 Fe(NO3)2 + N2 + 4HNO3
If the compound is used in situ, the compound is produced by the reaction of iron(II) chloride and calcium nitrate:[9][10]
- FeCl2 + Ca(NO3)2 → Fe(NO3)2 + CaCl2
Reactions
The hexahydrate melts at 60 °C and then decomposes at 61 °C into iron(III) oxide rather than iron(II) oxide.[1] A solution of iron(II) nitrate is much more stable, decomposing at 107 °C to iron(III), with the presence of nitric acid lowering the decomposition temperature. Concentrated nitric acid oxidizes iron(II) nitrate into iron(III) nitrate:[6]
- 3Fe(NO3)2 + 4HNO3 → 3Fe(NO3)3 + NO + 2H2O
Uses
Iron(II) nitrate has no uses, however, there is a potential use for dye removal.[10][9]
References
- ↑ 1.0 1.1 1.2 1.3 1.4 John Newton Friend (1921) (in en). Iron and Its Compounds. C. Griffin, Limited. pp. 175–176. https://books.google.com/books?id=EWQvAQAAMAAJ.
- ↑ Lide, David, ed (2004). "4" (in English) (Hardcover). CRC Handbook of Chemistry and Physics (85th ed.). Taylor & Francis. p. 62. ISBN 9780849304859. https://books.google.com/books?id=WDll8hA006AC.
- ↑ Herman Francis Mark; Anthony Standen (1963). Standen, Anthony. ed (in English). Encyclopedia of Chemical Technology. Interscience Publishers. https://books.google.com/books?id=a5zh2iclP-UC. Retrieved 16 February 2021.
- ↑ National Institute for Occupational Safety and Health (1981). Registry of Toxic Effects of Chemical Substances. U.S. Department of Health and Human Services, Public Health Service, Centers for Disease Control, National Institute for Occupational Safety and Health. p. 548. https://books.google.com/books?id=uGO1NK4cDJUC. Retrieved 16 February 2021.
- ↑ Hair, Neil J.; Beattie, James K. (1977). "Structure of Hexaaquairon(III) Nitrate Trihydrate. Comparison of Iron(II) and Iron(III) Bond Lengths in High-Spin Octahedral Environments". Inorganic Chemistry 16 (2): 245–250. doi:10.1021/ic50168a006.
- ↑ 6.0 6.1 Gus van Weert; Yuxing Shang (1993). "Iron control in nitrate hydrometallurgy by (auto) decomposition of iron (II) nitrate" (in en). Hydrometallurgy (Elsevier B.V.) 33 (3): 255–271. doi:10.1016/0304-386X(93)90066-M.
- ↑ Ludmila G.Lavrenova (2012). "Spin-crossover in the complex of iron(II) nitrate with tris(3,5-dimethylpyrazol-1-yl)methane" (in en). Inorganica Chimica Acta 382: 1–5. doi:10.1016/j.ica.2011.11.030.
- ↑ D. G. Karraker (1981) (in English) (PDF). Cu(II) – Catalyzed Hydrazine Reduction of Ferric Nitrate. United States Department of Energy. doi:10.2172/5658572. https://www.osti.gov/servlets/purl/5658572.
- ↑ 9.0 9.1 Arfin, Tanvir; Bhaisare, Dipti A.; Waghmare, S. S. (2021). "Development of a PANI/Fe(NO3)2 nanomaterial for reactive orange 16 (RO16) dye removal". Analytical Methods 13 (44): 5309–5327. doi:10.1039/D1AY01402A. PMID 34714901.
- ↑ 10.0 10.1 Muthusamy, Keerthana; Muzaffar, Aqib; M, Basheer Ahamed (2019). "Synthesis and characterization of Fe(NO3)2-NiO composite as a photocatalyst for degradation of methylene blue dye under UV-irradiation". Optik 177: 36–45. doi:10.1016/j.ijleo.2018.10.014. Bibcode: 2019Optik.177...36M.
| HNO3 | He | ||||||||||||||||
| LiNO3 | Be(NO3)2 | B(NO3)−4 | C | NO−3, NH4NO3 |
O | FNO3 | Ne | ||||||||||
| NaNO3 | Mg(NO3)2 | Al(NO3)3 | Si | P | S | ClONO2 | Ar | ||||||||||
| KNO3 | Ca(NO3)2 | Sc(NO3)3 | Ti(NO3)4 | VO(NO3)3 | Cr(NO3)3 | Mn(NO3)2 | Fe(NO3)3, Fe(NO3)2 |
Co(NO3)2, Co(NO3)3 |
Ni(NO3)2 | Cu(NO3)2 | Zn(NO3)2 | Ga(NO3)3 | Ge | As | Se | Br | Kr |
| RbNO3 | Sr(NO3)2 | Y(NO3)3 | Zr(NO3)4 | Nb | Mo | Tc | Ru | Rh | Pd(NO3)2 | AgNO3 | Cd(NO3)2 | In | Sn | Sb(NO3)3 | Te | I | Xe(NO3)2 |
| CsNO3 | Ba(NO3)2 | Hf | Ta | W | Re | Os | Ir | Pt | Au | Hg2(NO3)2, Hg(NO3)2 |
Tl(NO3)3, TlNO3 |
Pb(NO3)2 | Bi(NO3)3 BiO(NO3) |
Po | At | Rn | |
| FrNO3 | Ra(NO3)2 | Rf | Db | Sg | Bh | Hs | Mt | Ds | Rg | Cn | Nh | Fl | Mc | Lv | Ts | Og | |
| ↓ | |||||||||||||||||
| La(NO3)3 | Ce(NO3)3, Ce(NO3)4 |
Pr | Nd(NO3)3 | Pm | Sm | Eu(NO3)3 | Gd(NO3)3 | Tb(NO3)3 | Dy | Ho | Er | Tm | Yb | Lu | |||
| Ac(NO3)3 | Th(NO3)4 | Pa | UO2(NO3)2 | Np | Pu | Am | Cm | Bk | Cf | Es | Fm | Md | No | Lr | |||
 |


