Biology:Legionella pneumophila
| Legionella pneumophila | |
|---|---|
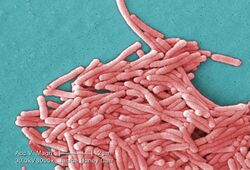
| |
| Colorized scanning electron micrograph image of L. pneumophila | |
| Scientific classification | |
| Domain: | Bacteria |
| Phylum: | Pseudomonadota |
| Class: | Gammaproteobacteria |
| Order: | Legionellales |
| Family: | Legionellaceae |
| Genus: | Legionella |
| Species: | L. pneumophila
|
| Binomial name | |
| Legionella pneumophila Brenner DJ, Steigerwalt AG, McDade JE 1979
| |
Legionella pneumophila is an aerobic, pleomorphic, flagellated, non-spore-forming, Gram-negative bacterium of the genus Legionella.[1][2] L. pneumophila is the primary human pathogenic bacterium in this group. In nature, L. pneumophila infects freshwater and soil amoebae of the genera Acanthamoeba and Naegleria.[3] This pathogen is found commonly near freshwater environments[4] and will then invade the amoebae found in these environments, using them to carry out metabolic functions.[5]
L. pneumophila is also the causative agent of Legionnaires' disease, also known as legionellosis. Due to L. pneumophila’s ability to thrive in water, it can grow in water filtration systems, leading to faucets, showers, and other fixtures and then spread through aerosolized water droplets.[5] Once infected, this bacterium can cause pneumonia with symptoms such as fever, delirium, diarrhea, and decreased liver and kidney function.[6]
After its initial discovery, it was found that Legionella replicates inside amoeba, which suggests that bacteria replicating in environmental protozoa have the ability to exploit signaling pathways in human phagocytic cells.[7]
Characterization
L. pneumophila is a coccobacillus. It is a Gram-negative, aerobic bacterium unable to hydrolyse gelatin or produce urease. It is also non-fermentative. L. pneumophila is neither pigmented nor does it autofluoresce. It is oxidase- and catalase-positive, and produces beta-lactamase. L. pneumophila colony morphology is gray-white with a textured, cut-glass appearance; it also requires cysteine and iron to thrive. It grows on yeast extract agar as well as in moist environments, such as tap water, in "opal-like" colonies.
Cell membrane structure
While L. pneumophila is categorized as a Gram-negative organism, it stains poorly due to its unique lipopolysaccharide content in the outer leaflet of the outer cell membrane.[8] The bases for the somatic antigen specificity of this organism are located on the side chains of its cell wall. The chemical composition of these side chains both with respect to components and arrangement of the different sugars, determines the nature of the somatic or O-antigenic determinants, which are important means of serologically classifying many Gram-negative bacteria. At least 35 different serovars of L. pneumophila have been described, as well as several other species subdivided into a number of serovars.[citation needed]
Ecology and reservoirs

L. pneumophila is a bacterium that can be found in numerous different environmental conditions. It can preside in temperatures ranging from 0-63 °C, a pH range of 5.0-8.5, and in dissolved oxygen levels of 0.2-15.0 mg/liter.[4]
In the environment, it can be found in freshwater environments within this wide range of temperatures. Although it can be found in this wider range, it only multiplies within a temperature of 25 and 42 °C.[4] With their ability to reside in water, they can also resist chlorination of water and pass into water control systems easily.[4] With this ability to infiltrate water systems, they can form biofilms in the walls of pipes which can lead to this bacterium being aerosolized through faucets, showers, sprinklers, and other fixtures, leading to infection after prolonged exposure.[5] The main cause of L. pneumophila contamination is the water supply network and this has allowed L. pneumophila to grow and proliferate in places such as cooling towers, water systems of hospitals, hotels, and cruise ships.[4]
As a facultative intracellular parasite, L. pneumophila can invade and replicate inside amoebae in the environment, especially within the species of the genera Acanthamoeba and Naegleria, which can thus serve as a reservoir for L. pneumophila. These hosts will then provide protection against unfavorable physical and chemical conditions, such as chlorination.[3]
Biofilms
Biofilms are specialized, surface attachment communities that can consist of one microbe, or multiple different ones, ranging from bacteria, algae, and protozoa. Biofilms on plumbing systems and in water distribution systems is where a lot of L. pneumophila can survive.[4] Between 2009 and 2010, L. pneumophila contributed to 58% of the total waterborne disease outbreaks associated with drinking water in the United States, causing an increase in research surrounding L. pneumophila biofilms and proliferation.[9] Material also plays a role in biofilm proliferation. In water piping, L. pneumophila was more commonly found in plastic pipes at 40 °C, rather than a copper pipe, which actually inhibited growth.[4]
Free-living protozoa
Legionella is a species that is known to infect and multiply within species of free-living amoeba. We know of at least 20 different species of free-living amoeba that support the intracellular replication of L. pneumophila. [10] This bacterium can infect and survive within the amoeba genera which includes: Acanthamoeba, Vermamoeba and Naegleria. L. pneumophila are surrounded by an amoeba-resistant cyst while residing inside the amoeba, allowing them to survive harsh environmental conditions such as chlorine, which is commonly used in water treatment systems.[11]
Although it is known that free-living amoeba play an important role in the ecology of L. pneumophila, there is little data on how these amoebas interact with other amoebas, and how this affects L. pneumophila overall.[10]
Frequency of occurrence
L. pneumophila is the primary (>90%) causative organism for Legionnaires disease.[12] Roughly 2 per 100,000 people are infected with Legionnaires disease each year in the EU.[13] L. pneumophila often infects individuals through poor quality water sources. Approximately 20% of reported Legionnaires disease cases come from healthcare, senior living, or travel facilities that have been exposed to water contaminated with L. pneumophila. [12] There may also be an increased risk of contracting L. pneumophila from private wells, as they are often unregulated and not as rigorously disinfected as municipal water systems.[14] Several large outbreaks of Legionnaire's Disease have come from public baths such as spas and hot tubs due to the temperature range of the water being ideal for Legionella growth.[15][16]
Pathogenesis
In humans, L. pneumophila invades and replicates inside macrophages. The internalization of the bacteria can be enhanced by the presence of antibody and complement, but is not absolutely required. Internalization of the bacteria appears to occur through phagocytosis. However, L. pneumophila is also capable of infecting non-phagocytic cells through an unknown mechanism. A rare form of phagocytosis known as coiling phagocytosis has been described for L. pneumophila, but this is not dependent on the Dot/Icm (intracellular multiplication/defect in organelle trafficking genes) bacterial secretion system and has been observed for other pathogens.[17] Once internalized, the bacteria surround themselves in a membrane-bound vacuole that does not fuse with lysosomes that would otherwise degrade the bacteria. In this protected compartment, the bacteria multiply.
Virulence factors
L. pneumophila exhibits a unique lipopolysaccharide (LPS) structure that is highly hydrophobic due to its being densely packed with branched fatty acids, and elevated levels of O-acetyl and N-acetyl groups.[18] This structure helps prevent interaction with a common LPS immune system co-receptor, CD14.[18] There is also a correlation between an LPS with a high molecular-weight and the inhibition of phagosome-lysosome fusion.[18] L. pneumophila produces pili of varying lengths. The two pili proteins: PilE and Prepilin peptidase (PilD) are responsible for the production of type IV pili and subsequently intracellular proliferation.[19] L. pneumophila possesses a singular, polar flagellum that is used for cell motility, adhesion, host invasion, and biofilm formation.[18] The same regulators that control flagellation also control lysosome avoidance and cytotoxicity.[18] The macrophage infectivity potentiator is another key component of host cell invasion and intracellular replication. MIP displays peptidyl–prolyl cis/trans isomerase (PPIase) activity which is crucial for survival within the macrophage, along with transmigration across the lung epithelial barrier.[18][19]
Dot/Icm type IV secretion system
The bacteria use a type IVB secretion system known as Dot/Icm to inject effector proteins into the host. These effectors are involved in increasing the bacteria's ability to survive inside the host cell. L. pneumophila encodes for over 330 "effector" proteins,[20] which are secreted by the Dot/Icm translocation system to interfere with host cell processes to aid bacterial survival. It has been predicted that the genus Legionella encodes more than 10,000 and possibly up to ~18,000 effectors that have a high probability to be secreted into their host cells.[21][22]
One main way in which L. pneumophila uses its effector proteins is to interfere with fusion of the Legionella-containing vacuole with the host's endosomes, and thus protect against lysis.[23] Knock-out studies of Dot/Icm translocated effectors indicate that they are vital for the intracellular survival of the bacterium, but many individual effector proteins are thought to function redundantly, in that single-effector knock-outs rarely impede intracellular survival. This high number of translocated effector proteins and their redundancy is likely a result of the bacterium having evolved in many different protozoan hosts.[24]
Legionella-containing vacuole
File:TEM image of Legionella pneumophila within a phagocytic cell.tif
For Legionella to survive within macrophages and protozoa, it must create a specialized compartment known as the Legionella-containing vacuole (LCV). Through the action of the Dot/Icm secretion system, the bacteria are able to prevent degradation by the normal endosomal trafficking pathway and instead replicate. Shortly after internalization, the bacteria specifically recruit endoplasmic reticulum-derived vesicles and mitochondria to the LCV while preventing the recruitment of endosomal markers such as Rab5a and Rab7a. Formation and maintenance of the vacuoles are crucial for pathogenesis; bacteria lacking the Dot/Icm secretion system are not pathogenic and cannot replicate within cells, while deletion of the Dot/Icm effector SdhA results in destabilization of the vacuolar membrane and no bacterial replication.[25][26]
Metabolism
L. pneumophila uses glycolysis, the Entner-Doudoroff (ED) pathway, the pentose phosphate pathway (PP), and the citric acid cycle (TCA).[27] Although L. pneumophila can also perform gluconeogenesis, it does not have the genes to encode for 1,6-biphosphatases. Therefore, other enzymes are used to complete gluconeogenesis. One enzyme used instead is fructose 6-phosphate aldolase.[27] This trend is also present when it comes to the PP pathway which can occur without substrates such as 6-phosphogluconate dehydrogenase.[27] The ED and PP pathways are the main pathways for glucose metabolism in this organism. Along with these pathways, serine was found to be a major nutrient due to its ability to be turned into pyruvate, which is an important intermediate in metabolic pathways in L. pneumophila.[27]
Although glucose metabolism is used, it is not one of the main synthesis pathways within the organism. While using media containing glucose, growth of L. pneumophila did not increase and carbohydrates were not considered an important carbon source within L. pneumophila. Glucose can act as a co-substrate only under certain conditions, as this microbe uses amino acids more frequently and efficiently.[27]
Nutrient acquisition
Legionella is auxotrophic for seven amino acids: cysteine, leucine, methionine, valine, threonine, isoleucine, and arginine. Once inside the host cell, Legionella needs nutrients to grow and reproduce. Inside the vacuole, nutrient availability is low; the high demand of amino acids is not covered by the transport of free amino acids found in the host cytoplasm. To improve the availability of amino acids, the parasite promotes the host mechanisms of proteasomal degradation. This process in L. pneumophila includes the SCF1 ubiquitin ligase and the AnkB F-Box effector, which is farnesylated by the activity of three host enzymes localized in the membrane of the LCV: farnesyltransferase, Ras-converting enzyme-1 protease, and ICMT. Farnesylation allows AnkB to get anchored into the cytoplasmic side of the vacuole. SCF1 and AnkB interact with each other to degrade Lys-linked polyubiquitinated proteins.[28] This generates an excess of free amino acids in the cytoplasm of L. pneumophila-infected cells that can be used for intravacuolar proliferation of the parasite.
The K48-linked polyubiquitination is a marker for proteasomal degradation that releases 2 to 24-amino-acid-long peptides, which are quickly degraded to amino acids by various oligopeptidases and aminopeptidases present in the cytoplasm. Amino acids are imported into the LCV through various amino acid transporters such as the neutral amino acid transporter B(0).[28]
The amino acids are the primary carbon and energy source of L. pneumophila, that have almost 12 classes of ABC-transporters, amino acid permeases, and many proteases, to exploit it. The imported amino acids are used by L. pneumophila to generate energy through the TCA cycle (Krebs cycle) and as sources of carbon and nitrogen. Because the amino acid degradation acts as the main carbon source for L. pneumophila, this microbe does not rely as heavily on glucose. Despite this, L. pneumophila does contain multiple amylases, such as LamB, which hydrolyzes polysaccharides into glucose monomers for metabolism. The loss of LamB can result in severe growth issues for L. pneumophila.[29]
However, promotion of proteasomal degradation for the obtention of amino acids and the hydrolyzation of polysaccharides may not be the only virulence strategies to obtain carbon and energy sources from the host. Type II–secreted degradative enzymes may provide an additional strategy to generate carbon and energy sources.[30] L. pneumophila is the only known intracellular pathogen to have a Type II Secretion System (secretome). In Type II Secretion, proteins are first translocated across the inner membrane into the periplasmic space. This process is mediated by either the Sec or Tat pathway. Soon after, the same proteins are then transported through a specific pore in the outer membrane to the exterior of the cell. This secretome is believed to have as many as 60 proteins incorporated into the system.[30]
Genomics
| NCBI genome ID | 416 |
|---|---|
| Ploidy | haploid |
| Genome size | 3.44 Mb |
| Number of chromosomes | 1 |
| Year of completion | 2004 |
The determination and publication of the complete genome sequences of three clinical L. pneumophila isolates in 2004 paved the way for the understanding of the molecular biology of L. pneumophila in particular and Legionella in general. In 2007, a fourth strain was discovered: L. pneumophila strain Corby.[31] These four strains resemble a very similar size, with strain Lens around 3.3 Mb and strain Paris and Corby about 3.5 Mb. This larger size reflects a higher number of genes, corresponding with the ability of Legionella to adapt to different hosts and environments. In all four strains of L. pneumophila, there is a relatively high abundance of eukaryotic-like proteins (ELPs), suggesting that these ELPs are beneficial for their everyday function. In fact, many of these ELPs are predicted to benefit the pathogen in modulating the host cell. In-depth comparative genome analysis using DNA arrays to study the gene content of 180 Legionella strains revealed high genome plasticity and frequent horizontal gene transfer. Further insight in the L. pneumophila lifecycle was gained by investigating the gene expression profile of L. pneumophila in Acanthamoeba castellanii, its natural host. L. pneumophila exhibits a biphasic lifecycle and defines transmissive and replicative traits according to gene expression profiles.[2]
Genetic transformation
Transformation is a bacterial adaptation involving the transfer of DNA from one bacterium to another through the surrounding liquid medium. Transformation is a bacterial form of sexual reproduction.[32] In order for a bacterium to bind, take up, and recombine exogenous DNA into its chromosome, it must enter a special physiological state referred to as "competence".
To determine which molecules may induce competence in L. pneumophila, 64 toxic molecules were tested.[33] Only six of these molecules, all DNA-damaging agents, caused strong induction of competence. These were mitomycin C (which introduces DNA inter-strand crosslinks), norfloxacin, ofloxacin, and nalidixic acid (inhibitors of DNA gyrase that cause double-strand breaks), bicyclomycin (causes double-strand breaks), and hydroxyurea (causes oxidation of DNA bases). These results suggest that competence for transformation in L. pneumophila evolved as a response to DNA damage.[33] Perhaps induction of competence provides a survival advantage in a natural host, as occurs with other pathogenic bacteria.[32]
Drug targets
Several enzymes in the bacteria have been proposed as tentative drug targets. For example, enzymes in the iron uptake pathway have been suggested as important drug targets.[34] Further, a cN-II class of IMP/GMP specific 5´-nucleotidase which has been extensively characterized kinetically. The tetrameric enzyme shows aspects of positive homotropic cooperativity, substrate activation and presents a unique allosteric site that can be targeted to design effective drugs against the enzyme and thus, the organism. Moreover, the enzyme is distinct than its human counterpart making it an attractive target for drug development.
Detection and treatment
Antisera have been used both for slide agglutination studies and for direct detection of bacteria in tissues using immunofluorescence via fluorescent-labelled antibody. Specific antibody in patients can be determined by the indirect fluorescent antibody test. ELISA and microagglutination tests have also been successfully applied.[35]
Legionella stains poorly with Gram stain, stains positive with silver, and is cultured on charcoal yeast extract with iron and cysteine.[citation needed] A consistent method that has been used to detect the disease is the urine antigen test.[36]
Effective antibiotic treatment for Legionella pneumonia includes fluoroquinolones (levofloxacin or moxifloxacin) or macrolides ( preferably azithromycin).[36] There has been no significant difference found between using a fluoroquinolone or a macrolide to treat Legionella pneumonia.[36] Combination treatments with rifampicin are being tested as a response to antibiotic resistance during monotreatments, though its effectiveness remains uncertain.[36]
These antibiotics work best because L. pneumophila is an intracellular pathogen.[37] Fluoroquinolones and macrolides have great intracellular activity and are able to penetrate into Legionella-infected cells. The Infectious Diseases Society of America recommends 5–10 days of treatment with levofloxacin or 3–5 days of treatment with azithromycin, however patients that are immunocompromised or have a severe disease may require an extended course of treatment.[37]
References
- ↑ Brock Biology of Microorganisms (11th ed.). Prentice Hall. 2005. ISBN 0-13-144329-1.
- ↑ 2.0 2.1 Legionella: Molecular Microbiology. Caister Academic Press. 2008. ISBN 978-1-904455-26-4. http://www.horizonpress.com/leg.
- ↑ 3.0 3.1 "Preliminary report on the pathogenicity of Legionella pneumophila for freshwater and soil amoebae". Journal of Clinical Pathology 33 (12): 1179–1183. December 1980. doi:10.1136/jcp.33.12.1179. PMID 7451664.
- ↑ 4.0 4.1 4.2 4.3 4.4 4.5 4.6 Legionella Pneumophila: From Environment to Disease. NOVA Science Publishers. 2010. pp. 5–8. ISBN 978-1-60876-947-6. https://hdl.handle.net/11454/19520.
- ↑ 5.0 5.1 5.2 "Biofilms: the stronghold of Legionella pneumophila". International Journal of Molecular Sciences 14 (11): 21660–21675. October 2013. doi:10.3390/ijms141121660. PMID 24185913.
- ↑ "An outbreak of Legionnaires' disease in newborns in Serbia". Paediatrics and International Child Health 42 (2): 59–66. May 2022. doi:10.1080/20469047.2022.2108672. PMID 35944175.
- ↑ "Genome dynamics in Legionella: the basis of versatility and adaptation to intracellular replication". Cold Spring Harbor Perspectives in Medicine 3 (6): a009993. June 2013. doi:10.1101/cshperspect.a009993. PMID 23732852.
- ↑ "Chapter 26: Legionella". Sherris Medical Microbiology (4th ed.). McGraw Hill. 2004. ISBN 978-0-8385-8529-0.
- ↑ "Role of biofilm roughness and hydrodynamic conditions in Legionella pneumophila adhesion to and detachment from simulated drinking water biofilms". Environmental Science & Technology 49 (7): 4274–4282. April 2015. doi:10.1021/es505842v. PMID 25699403. Bibcode: 2015EnST...49.4274S.
- ↑ 10.0 10.1 "Impact of inter-amoebic phagocytosis on the L. pneumophila growth". FEMS Microbiology Letters 367 (18). September 2020. doi:10.1093/femsle/fnaa147. PMID 32860684.
- ↑ "Detection of amoeba-associated Legionella pneumophila in hospital water networks of Johannesburg.". Southern African Journal of Infectious Diseases 33 (3): 72–75. 2018. doi:10.1080/23120053.2018.1434060.
- ↑ 12.0 12.1 "Water quality influences Legionella pneumophila determination". Water Research 238: 119989. June 2023. doi:10.1016/j.watres.2023.119989. PMID 37137207. Bibcode: 2023WatRe.23819989D.
- ↑ "Legionella pneumophila Infections during a 7-Year Retrospective Analysis (2016-2022): Epidemiological, Clinical Features and Outcomes in Patients with Legionnaires' Disease". Microorganisms 11 (2): 498. February 2023. doi:10.3390/microorganisms11020498. PMID 36838463.
- ↑ "Legionella pneumophila occurrence in drinking water supplied by private wells". Letters in Applied Microbiology 70 (4): 232–240. April 2020. doi:10.1111/lam.13273. PMID 31904109.
- ↑ "Outbreak of Legionnaire's Disease Caused by Legionella pneumophila Serogroups 1 and 13". Emerging Infectious Diseases 23 (2): 349–351. February 2017. doi:10.3201/eid2302.161012. PMID 28098535.
- ↑ "Legionnaires' Disease Outbreak Associated With a Hot Tub Display at the North Carolina Mountain State Fair, September 2019". Public Health Reports: 333549231159159. March 2023. doi:10.1177/00333549231159159. PMID 36971250.
- ↑ "Coiling phagocytosis is the preferential phagocytic mechanism for Borrelia burgdorferi". Infection and Immunity 60 (10): 4205–4212. October 1992. doi:10.1128/iai.60.10.4205-4212.1992. PMID 1398932.
- ↑ 18.0 18.1 18.2 18.3 18.4 18.5 "Virulence properties of the legionella pneumophila cell envelope". Frontiers in Microbiology 2: 74. 2011. doi:10.3389/fmicb.2011.00074. PMID 21747794.
- ↑ 19.0 19.1 "Legionella pneumophila-Virulence Factors and the Possibility of Infection in Dental Practice". Microorganisms 10 (2): 255. January 2022. doi:10.3390/microorganisms10020255. PMID 35208710.
- ↑ "Legionella pneumophila, armed to the hilt: justifying the largest arsenal of effectors in the bacterial world". Current Opinion in Microbiology 29: 74–80. February 2016. doi:10.1016/j.mib.2015.11.002. PMID 26709975.
- ↑ "Genomic analysis of 38 Legionella species identifies large and diverse effector repertoires". Nature Genetics 48 (2): 167–175. February 2016. doi:10.1038/ng.3481. PMID 26752266.
- ↑ "More than 18,000 effectors in the Legionella genus genome provide multiple, independent combinations for replication in human cells". Proceedings of the National Academy of Sciences of the United States of America 116 (6): 2265–2273. February 2019. doi:10.1073/pnas.1808016116. PMID 30659146. Bibcode: 2019PNAS..116.2265G.
- ↑ "Ankyrin repeat proteins comprise a diverse family of bacterial type IV effectors". Science 320 (5883): 1651–1654. June 2008. doi:10.1126/science.1158160. PMID 18566289. Bibcode: 2008Sci...320.1651P.
- ↑ "Legionella pneumophila adaptation to intracellular life and the host response: clues from genomics and transcriptomics". FEBS Letters 581 (15): 2829–2838. June 2007. doi:10.1016/j.febslet.2007.05.026. PMID 17531986.
- ↑ "The Dot/Icm effector SdhA is necessary for virulence of Legionella pneumophila in Galleria mellonella and A/J mice". Infection and Immunity 81 (7): 2598–2605. July 2013. doi:10.1128/IAI.00296-13. PMID 23649096.
- ↑ "The protein SdhA maintains the integrity of the Legionella-containing vacuole". Proceedings of the National Academy of Sciences of the United States of America 109 (9): 3481–3486. February 2012. doi:10.1073/pnas.1121286109. PMID 22308473.
- ↑ 27.0 27.1 27.2 27.3 27.4 "The life stage-specific pathometabolism of Legionella pneumophila". FEBS Letters 590 (21): 3868–3886. November 2016. doi:10.1002/1873-3468.12326. PMID 27455397.
- ↑ 28.0 28.1 "Nutrient generation and retrieval from the host cell cytosol by intra-vacuolar Legionella pneumophila". Frontiers in Cellular and Infection Microbiology 4: 111. 2014-08-26. doi:10.3389/fcimb.2014.00111. PMID 25207263.
- ↑ "A Legionella pneumophila amylase is essential for intracellular replication in human macrophages and amoebae". Scientific Reports 8 (1): 6340. April 2018. doi:10.1038/s41598-018-24724-1. PMID 29679057. Bibcode: 2018NatSR...8.6340B.
- ↑ 30.0 30.1 "Gene transfer in Legionella pneumophila". Microbes and Infection 1 (14): 1203–1209. December 1999. doi:10.1016/s1286-4579(99)00241-5. PMID 10580276.
- ↑ "Legionella pathogenicity: genome structure, regulatory networks and the host cell response". International Journal of Medical Microbiology. Special issue: Pathogenomics 297 (7–8): 577–587. November 2007. doi:10.1016/j.ijmm.2007.03.009. PMID 17467337.
- ↑ 32.0 32.1 "Adaptive value of sex in microbial pathogens". Infection, Genetics and Evolution 8 (3): 267–285. May 2008. doi:10.1016/j.meegid.2008.01.002. PMID 18295550.
- ↑ 33.0 33.1 "Antibiotics and UV radiation induce competence for natural transformation in Legionella pneumophila". Journal of Bacteriology 193 (5): 1114–1121. March 2011. doi:10.1128/JB.01146-10. PMID 21169481.
- ↑ "An update on iron acquisition by Legionella pneumophila: new pathways for siderophore uptake and ferric iron reduction". Future Microbiology 10 (5): 841–851. May 2015. doi:10.2217/fmb.15.21. PMID 26000653.
- ↑ "Specific antisera and immunological procedures for characterization of methanogenic bacteria". Journal of Bacteriology 149 (1): 320–328. January 1982. doi:10.1128/jb.149.1.320-328.1982. PMID 6172417.
- ↑ 36.0 36.1 36.2 36.3 "Legionnaires' Disease: Update on Diagnosis and Treatment". Infectious Diseases and Therapy 11 (3): 973–986. June 2022. doi:10.1007/s40121-022-00635-7. PMID 35505000.
- ↑ 37.0 37.1 "Legionnaires' disease". Lancet 387 (10016): 376–385. January 2016. doi:10.1016/s0140-6736(15)60078-2. PMID 26231463.
External links
- Major topic "Legionella pneumophila": free-full text articles in PubMed
- Legionella pneumophila, causative agent of Legionnaire's disease and Pontiac fever at MetaPathogen
- Type strain of Legionella pneumophila at BacDive - the Bacterial Diversity Metadatabase
Wikidata ☰ Q147885 entry
 |


