Biology:Transforming growth factor beta family
| Transforming growth factor beta like domain | |||||||||
|---|---|---|---|---|---|---|---|---|---|
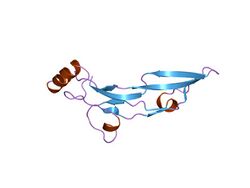 Structure of human transforming growth factor-beta 2.[1] | |||||||||
| Identifiers | |||||||||
| Symbol | TGF_beta | ||||||||
| Pfam | PF00019 | ||||||||
| Pfam clan | CL0079 | ||||||||
| InterPro | IPR001839 | ||||||||
| PROSITE | PDOC00223 | ||||||||
| SCOP2 | 1tfg / SCOPe / SUPFAM | ||||||||
| |||||||||
The transforming growth factor beta (TGF-β) family is a large group of structurally related cell regulatory proteins that was named after its first member, TGF-β1, originally described in 1983.[2] They interact with TGF-beta receptors.
Many proteins have since been described as members of the TGF-β family in a variety of species, including invertebrates as well as vertebrates and categorized into 23 distinct gene types that fall into four major subfamilies:[3][4][5]
- The TGF-β subfamily
- The bone morphogenetic proteins and the growth differentiation factors
- The activin and inhibin subfamilies
- The left-right determination factors
- A group encompassing various divergent members
Transforming growth factor-beta (TGF-beta)[6] is a multifunctional peptide that controls proliferation, differentiation and other functions in many cell types. TGF-beta-1 is a peptide of 112 amino acid residues derived by proteolytic cleavage from the C-terminal of a precursor protein. These proteins interact with a conserved family of cell surface serine/threonine-specific protein kinase receptors, and generate intracellular signals using a conserved family of proteins called SMADs. They play fundamental roles in the regulation of basic biological processes such as growth, development, tissue homeostasis and regulation of the immune system.[3]
Structure
Proteins from the TGF-beta family are only active as homo- or heterodimer; the two chains being linked by a single disulfide bond. From X-ray studies of TGF-beta-2,[7] it is known that all the other cysteines are involved in intrachain disulfide bonds. As shown in the following schematic representation, there are four disulfide bonds in the TGF-beta's and in inhibin beta chains, while the other members of this family lack the first bond.
interchain
|
+------------------------------------------|+
| ||
| | | | | |
+------+ +--|----------------------------------------+ |
+------------------------------------------+
where 'C' denotes a conserved cysteine involved in a disulfide bond.
Examples
Human genes encoding proteins that contain this domain include:
AMH; ARTN; BMP2; BMP3; BMP4; BMP5; BMP6; BMP7; BMP8A; BMP8B; BMP10; BMP15; GDF1; GDF2; GDF3; GDF5; GDF6; GDF7; GDF9; GDF10; GDF11; GDF15; GDNF; INHA; INHBA; INHBB; INHBC; INHBE; LEFTY1; LEFTY2; MSTN; NODAL; NRTN; PSPN; TGFB1; TGFB2; TGFB3;
References
- ↑ "An unusual feature revealed by the crystal structure at 2.2 A resolution of human transforming growth factor-beta 2". Nature 358 (6385): 430–4. July 1992. doi:10.1038/358430a0. PMID 1641027. Bibcode: 1992Natur.358..430S.
- ↑ "Transforming growth factor-beta in human platelets. Identification of a major storage site, purification, and characterization". J. Biol. Chem. 258 (11): 7155–60. June 1983. doi:10.1016/S0021-9258(18)32345-7. PMID 6602130.
- ↑ 3.0 3.1 "Transforming growth factor-beta-related proteins: an ancestral and widespread superfamily of cytokines in metazoans". Dev. Comp. Immunol. 28 (5): 461–85. May 2004. doi:10.1016/j.dci.2003.09.007. PMID 15062644.
- ↑ Burt DW (April 1992). "Evolutionary grouping of the transforming growth factor-beta superfamily". Biochem. Biophys. Res. Commun. 184 (2): 590–5. doi:10.1016/0006-291X(92)90630-4. PMID 1575734.
- ↑ "Evolution of the transforming growth factor-beta superfamily". Prog. Growth Factor Res. 5 (1): 99–118. 1994. doi:10.1016/0955-2235(94)90020-5. PMID 8199356. https://www.pure.ed.ac.uk/ws/files/11784469/1_s2.0_0955223594900205_main.pdf.
- ↑ Peptide growth factors and their receptors. Berlin: Springer-Verlag. 1990. ISBN 3-540-51184-9. https://archive.org/details/peptidegrowthfac0095unse_pt02.
- ↑ "Crystal structure of transforming growth factor-beta 2: an unusual fold for the superfamily". Science 257 (5068): 369–73. July 1992. doi:10.1126/science.1631557. PMID 1631557. Bibcode: 1992Sci...257..369D. https://zenodo.org/record/1230942.

