Chemistry:Lanthanum oxide

| |
| |
| Names | |
|---|---|
| IUPAC name
Lanthanum(III) oxide
| |
| Other names
Lanthanum sesquioxide
Lanthana | |
| Identifiers | |
3D model (JSmol)
|
|
| ChemSpider | |
| EC Number |
|
PubChem CID
|
|
| RTECS number |
|
| UNII | |
| |
| |
| Properties | |
| La2O3 | |
| Molar mass | 325.808 g·mol−1 |
| Appearance | White powder, hygroscopic |
| Density | 6.51 g/cm3, solid |
| Melting point | 2,315 °C (4,199 °F; 2,588 K) |
| Boiling point | 4,200 °C (7,590 °F; 4,470 K) |
| Insoluble | |
| Band gap | 4.3 eV |
| −78.0·10−6 cm3/mol | |
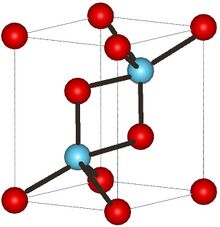
| Structure | |
| Hexagonal, hP5 | |
| P-3m1, No. 164 | |
| Hazards | |
| Main hazards | Irritant |
| Safety data sheet | External SDS |
| GHS pictograms |  [1] [1]
|
| GHS Signal word | Warning[1] |
| H315, H319, H335[1] | |
| P261, P280, P301+310, P304+340, P305+351+338, P405, P501[1] | |
| NFPA 704 (fire diamond) | |
| Flash point | Non-flammable |
| Related compounds | |
Other anions
|
Lanthanum(III) chloride |
Other cations
|
Cerium(III) oxide Actinium(III) oxide |
Related compounds
|
Lanthanum aluminium oxide, LaSrCoO4 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Lanthanum(III) oxide, also known as lanthana, chemical formula La
2O
3, is an inorganic compound containing the rare earth element lanthanum and oxygen. It is used in some ferroelectric materials, as a component of optical materials, and is a feedstock for certain catalysts, among other uses.
Properties

2O
3 powder
Lanthanum oxide is a white solid that is insoluble in water, but dissolves in acidic solutions. La
2O
3 absorbs moisture from air, converts to lanthanum hydroxide.[2]
Lanthanum oxide has p-type semiconducting properties and a band gap of approximately 5.8 eV.[3] Its average room temperature resistivity is 10 kΩ·cm, which decreases with an increase in temperature. La
2O
3 has the lowest lattice energy of the rare earth oxides, with very high dielectric constant, ε = 27.
Structure
At low temperatures, La
2O
3 has an A-M
2O
3 hexagonal crystal structure. The La3+ metal atoms are surrounded by a 7 coordinate group of O2− atoms, the oxygen ions are in an octahedral shape around the metal atom and there is one oxygen ion above one of the octahedral faces.[4] On the other hand, at high temperatures lanthanum oxide converts to a C-M
2O
3 cubic crystal structure. The La3+ ion is surrounded by six O2− ions in a hexagonal configuration.[5][6]
Synthesis
Lanthanum oxide can crystallize in at least three polymorphs.[2]
Hexagonal La
2O
3 has been produced by spray pyrolysis of lanthanum chloride.[7]
- 2 LaCl
3 + 3 H
2O → La(OH)
3 + 3 HCl - 2 La(OH)
3 → La
2O
3 + 3 H
2O
An alternative route to obtaining hexagonal La
2O
3 involves precipitation of nominal La(OH)
3 from aqueous solution using a combination of 2.5% NH
3 and the surfactant sodium dodecyl sulfate followed by heating and stirring for 24 hours at 80 °C:
- 2 LaCl
3 + 3 H
2O + 3 NH
3 → La(OH)
3 + 3 [NH
4]Cl
Other routes include:
- 2 La
2S
3 + 3 CO
2 → 2 La
2O
3 + 3 CS
2
Reactions
Lanthanum oxide is used as an additive to develop certain ferroelectric materials, such as La-doped bismuth titanate (Bi
4Ti
3O
12 - BLT).
Lanthanum oxide is used in optical materials; often the optical glasses are doped with La
2O
3 to improve the glass' refractive index, chemical durability, and mechanical strength.[8]
- 3 B
2O
3 + La
2O
3 → 2 La(BO
2)
3[clarification needed]
The addition of the La
2O
3 to the glass melt leads to a higher glass transition temperature from 658 °C to 679 °C. The addition also leads to a higher density, microhardness, and refractive index of the glass.
Potential applications
Lanthanum oxide is most useful as a precursor to other lanthanum compounds.[9] Neither the oxide nor any of the derived materials enjoys substantial commercial value, unlike some of the other lanthanides. Many reports describe efforts toward practical applications of La
2O
3, as described below.
La
2O
3 forms glasses of high density, refractive index, and hardness. Together with oxides of tungsten, tantalum, and thorium, La
2O
3 improves the resistance of the glass to attack by alkali. La
2O
3 is an ingredient in some piezoelectric and thermoelectric materials.
La
2O
3 has been examined for the oxidative coupling of methane.[10]
References
- ↑ 1.0 1.1 1.2 1.3 "Lanthanum Oxide". American Elements. https://www.americanelements.com/lanthanum-oxide-1312-81-8.
- ↑ 2.0 2.1 Greenwood, Norman N.; Earnshaw, Alan (1997). Chemistry of the Elements (2nd ed.). Butterworth-Heinemann. ISBN 978-0-08-037941-8.
- ↑ Shang, G.; Peacock, P. W.; Robertson, J. (2004). "Stability and band offsets of nitrogenated high-dielectric-constant gate oxides". Applied Physics Letters 84 (1): 106–108. doi:10.1063/1.1638896. Bibcode: 2004ApPhL..84..106S.
- ↑ Wells, A.F. (1984). Structural Inorganic Chemistry. Oxford: Clarendon Press. p. 546.
- ↑ Wyckoff, R. W.G. (1963). Crystal Structures: Inorganic Compounds RXn, RnMX2, RnMX3. New York: Interscience Publishers.
- ↑ Adachi, Gin-ya; Imanaka, Nobuhito (1998). "The Binary Rare Earth Oxides". Chemical Reviews 98 (4): 1479–1514. doi:10.1021/cr940055h. PMID 11848940.
- ↑ Kale, S.S.; Jadhav, K.R.; Patil, P.S.; Gujar, T.P.; Lokhande, C.D. (2005). "Characterizations of spray-deposited lanthanum oxide (La2O3) thin films". Materials Letters 59 (24–25): 3007–3009. doi:10.1016/j.matlet.2005.02.091.
- ↑ Vinogradova, N. N.; Dmitruk, L. N.; Petrova, O. B. (2004). "Glass Transition and Crystallization of Glasses Based on Rare-Earth Borates". Glass Physics and Chemistry 30: 1–5. doi:10.1023/B:GPAC.0000016391.83527.44.
- ↑ "Lanthanum has also found modest uses." Greenwood, Norman N.; Earnshaw, Alan (1997). Chemistry of the Elements (2nd ed.). Butterworth-Heinemann. p. 946. ISBN 978-0-08-037941-8.
- ↑ Manoilova, O.V. (2004). "Surface acidity and basicity of La2O3, LaOCl, and LaCl3 characterized by IR spectroscopy, TPD, and DFT calculations". J. Phys. Chem. B 108 (40): 15770–15781. doi:10.1021/jp040311m.
 |

