Chemistry:Zomepirac
 | |
| Clinical data | |
|---|---|
| Routes of administration | By mouth |
| ATC code | |
| Legal status | |
| Legal status |
|
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| ChEBI | |
| ChEMBL | |
| Chemical and physical data | |
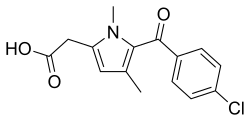
| Formula | C15H14ClNO3 |
| Molar mass | 291.73 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| | |
Zomepirac is an orally effective nonsteroidal anti-inflammatory drug (NSAID) that has antipyretic actions. It was developed by McNeil Pharmaceutical, approved by the FDA in 1980, and sold as the sodium salt zomepirac sodium, under the brand name Zomax. Due to its clinical effectiveness, it was preferred by doctors in many situations and obtained a large share of the analgesics market; however, it was subsequently withdrawn in March 1983 due to its tendency to cause serious anaphylaxis in a small, but unpredictable, subset of the patient population.[1][2]
Indications
Zomepirac was indicated for the management of mild to severe pain.[3] Multiple clinical trials demonstrated zomepirac to be more effective than aspirin or codeine alone and to be as effective as analgesic combinations containing codeine or other opioids.[4][5][6][7][8][9][10] Zomepirac provided analgesia comparable with usual intramuscular doses of morphine in postoperative pain and that with long-term use, neither tolerance to its analgesic effect nor psychological or physical dependence had been demonstrated.[3][11]
Chemical structure
Zomepirac is the sodium salt of 5-(4-chlorobenzoyl)-1,4 dimethyl-1H-pyrrole-2-acetate dihydrate. It is a pyrrole-acetic acid which is structurally related to tolmetin. The chemical structure differs from other NSAIDs in that the central benzene ring has been replaced by a pyrrole.
Mechanism of action
Zomepirac is a prostaglandin synthetase inhibitor.[12]
Anaphylaxis
Zomepirac does not cause anaphylaxis directly, but it is metabolized by UDP-glucuronosyltransferase (UGT) to a reactive glucuronide which binds irreversibly to plasma albumin.[13]
Synthesis
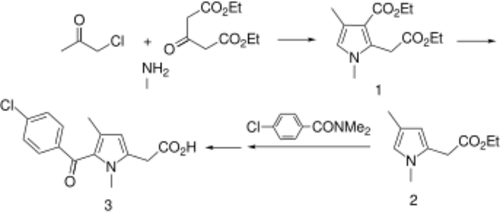
Zomepirac can be synthesized from diethyl 1,3-acetonedicarboxylate, chloroacetone, and aqueous methylamine (MeNH2) via modification of the Hantzsch pyrrole synthesis to give intermediate 1. Saponification, monoesterification, and thermal decarboxylation gives ester 2. This is acylated with N,N-dimethyl-p-chlorobenzamide, and finally saponification gives zomepirac (3).
See also
References
- ↑ "Reporting of adverse drug events: a key to postmarketing drug safety". American Family Physician 46 (3): 873–874. September 1992. PMID 1514478. http://www.findarticles.com/p/articles/mi_m3225/is_n3_v46/ai_12645044.
- ↑ "Identification of zomepirac-S-acyl-glutathione in vitro in incubations with rat hepatocytes and in vivo in rat bile". Drug Metabolism and Disposition 31 (11): 1429–1436. November 2003. doi:10.1124/dmd.31.11.1429. PMID 14570776.
- ↑ 3.0 3.1 "Zomepirac sodium. A new nonaddicting analgesic". JAMA 246 (4): 377–379. 1981. doi:10.1001/jama.246.4.377. PMID 7241789.
- ↑ "A multi-centre study of zomepirac in painful conditions: an analysis of clinical data for 15,484 patients". Current Medical Research and Opinion 8 (6): 382–391. 1983. doi:10.1185/03007998309111743. PMID 6221886.
- ↑ "Zomepirac sodium vs APC with codeine for oral surgery pain". Journal of Oral Surgery 39 (6): 426–429. June 1981. PMID 7014804.
- ↑ "Analgesic efficacy of zomepirac sodium in patients with pain due to cancer". Journal of Clinical Pharmacology 21 (11): 501–507. 1981. doi:10.1002/j.1552-4604.1981.tb05657.x. PMID 7037868.
- ↑ "Zomepirac, placebo and paracetamol/dextropropoxyphene combination compared in orthopaedic postoperative pain". British Journal of Anaesthesia 54 (9): 927–933. September 1982. doi:10.1093/bja/54.9.927. PMID 7052110.
- ↑ "Comparison of zomepirac, APC with codeine, codeine and placebo in the treatment of moderate and severe postoperative pain". Journal of Clinical Pharmacology 20 (4): 243–249. April 1980. doi:10.1002/j.1552-4604.1980.tb01704.x. PMID 6991540.
- ↑ "Clinical comparison of zomepirac with APC/codeine combination in the treatment of pain following oral surgery". Journal of Clinical Pharmacology 20 (4): 271–278. April 1980. doi:10.1002/j.1552-4604.1980.tb01708.x. PMID 6991544.
- ↑ "A double-blind study of zomepirac sodium and placebo in the treatment of muscle contraction headache". Headache 21 (2): 45–48. March 1981. doi:10.1111/j.1526-4610.1981.hed2102045.x. PMID 7016809.
- ↑ "Relative analgesic potency of oral zomepirac and intramuscular morphine in cancer patients with postoperative pain". Journal of Clinical Pharmacology 20 (4): 250–258. April 1980. doi:10.1002/j.1552-4604.1980.tb01705.x. PMID 6991541.
- ↑ DC McLeod, Zomepirac (Zomax, McNeil Pharmaceutical) , Drug Intelligence & Clinical Pharmacy: Vol. 15, No. 7, pp. 522-530.
- ↑ "Irreversible binding of zomepirac to plasma protein in vitro and in vivo". The Journal of Clinical Investigation 77 (3): 934–939. March 1986. doi:10.1172/JCI112392. PMID 3949982.
- ↑ "5-Benzoyl-1-methylpyrrole-2-acetic acids as antiinflammatory agents. 2. The 4-methyl compounds". Journal of Medicinal Chemistry 16 (2): 172–174. February 1973. doi:10.1021/jm00260a023. PMID 4683116.
- ↑ Carson JR, "5-Aroylpyrrole [5-aroyl pyrroles]", DE patent 2102746, published 1971-08-12
- ↑ Carson JS, US patent 3752826, issued 1973, assigned to McNeil
 |

