Chemistry:Nabumetone
 | |
| Clinical data | |
|---|---|
| Trade names | Relafen |
| AHFS/Drugs.com | Monograph |
| MedlinePlus | a692022 |
| License data |
|
| Routes of administration | By mouth |
| ATC code | |
| Legal status | |
| Legal status | |
| Pharmacokinetic data | |
| Protein binding | > 99% (active metabolite) |
| Metabolism | Liver, to active metabolite 6-methoxy-2-naphthylacetic acid; 6-MNA |
| Elimination half-life | 23 hours (active metabolite) |
| Excretion | Kidney |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| IUPHAR/BPS | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEBI | |
| ChEMBL | |
| Chemical and physical data | |
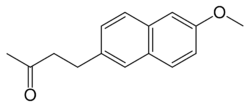
| Formula | C15H16O2 |
| Molar mass | 228.291 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| | |

Nabumetone, sold under the brand name Relafen among others, is a nonsteroidal anti-inflammatory drug (NSAID).[2] Nabumetone was developed by Beecham and first received regulatory approval in 1991.[3]
Nabumetone is a non-acidic NSAID prodrug that is rapidly metabolized in the liver to the active metabolite, 6-methoxy-2-naphthyl acetic acid. Nabumetone's active metabolite inhibits the cyclooxygenase enzyme and preferentially blocks COX-2 activity (which is indirectly responsible for the production of inflammation and pain during arthritis). The active metabolite of nabumetone is felt to be the compound primarily responsible for therapeutic effect. Comparatively, the parent drug is a poor inhibitor of COX-2 byproducts, particularly prostaglandins. It may be less nephrotoxic than indomethacin.[4] There are two known polymorphs of the compound.[5] Nabumetone has little effect on renal prostaglandin secretion and less of an association with heart failure than other traditional drugs of the class.[6] Effects of nabumetone on blood pressure control in hypertensive patients on ACE inhibitors are also good,[clarification needed] equivalent to paracetamol.[7]
In 2021, it was the 250th most commonly prescribed medication in the United States, with more than 1 million prescriptions.[8][9]
Medical uses
Nabumetone is used to treat pain and inflammation.[citation needed]
Side effects
It has been shown to have a slightly lower risk of gastrointestinal side effects than most other nonselective NSAIDs, since it is a non-acidic prodrug that is metabolized to its active 6-MNA (6-methoxy-2-naphthylacetic acid) form.[citation needed]
Side effects include bloody or black, tarry stools; change in color, frequency, or amount of urine; chest pain; shortness of breath; coughing up blood; pale stools; numbness; weakness; flu-like symptoms; leg pain; vision problems; speech problems; problems walking; weight gain; stomach pain; cold sweat; skin rash; blisters; headache; swelling; bleeding; bruising; vomiting blood; jaundice; diarrhea; constipation; dizziness; indigestion; gas; nausea; and ringing in the ears.[10]
In October 2020, the U.S. Food and Drug Administration (FDA) required the drug label to be updated for all nonsteroidal anti-inflammatory medications to describe the risk of kidney problems in unborn babies that result in low amniotic fluid.[11][12] They recommend avoiding NSAIDs in pregnant women at 20 weeks or later in pregnancy.[11][12]
Society and culture
Brand names
It is available under numerous brand names, including Relafen, Relifex, and Gambaran.
References
- ↑ "Carbon-carbon bond cleavage in activation of the prodrug nabumetone". Drug Metabolism and Disposition 42 (5): 828–838. May 2014. doi:10.1124/dmd.114.056903. PMID 24584631.
- ↑ "Severe immediate reaction to nabumetone". Journal of Investigational Allergology & Clinical Immunology 17 (4): 274–276. 2007. PMID 17694703.
- ↑ "Nabumetone Page". RCSB Protein Data Bank. https://www.rcsb.org/ligand/NBO.
- ↑ "Non-steroidal anti-inflammatory drugs and renal response to exercise: a comparison of indomethacin and nabumetone". Clinical Science 97 (4): 457–465. October 1999. doi:10.1042/cs0970457. PMID 10491346.
- ↑ "Polymorphism of Nabumetone". Crystal Growth & Design 2 (6): 501–503. 2002. doi:10.1021/cg0255568.
- ↑ "098. A Drug-Safety Study to Examine the Possible Association of Congestive Heart Failure with Dispensed Nabumetone, Ibuprofen and other Non-Steroidal Anti-inflammatory Drugs". Pharmacoepidemiology and Drug Safety 8 (S2): S115. 2000. doi:10.1002/(SICI)1099-1557(199908)8:2+<S79::AID-PDS429>3.0.CO;2-2.
- ↑ "Effects of ibuprofen (IB), nabumetone (N) and celecoxib (C) on blood pressure (BP) control in hypertensive patients on ACE inhibitors". American Journal of Hypertension 14 (S1): 85A. 2001. doi:10.1016/S0895-7061(01)01811-8.
- ↑ "The Top 300 of 2021". https://clincalc.com/DrugStats/Top300Drugs.aspx.
- ↑ "Nabumetone - Drug Usage Statistics". https://clincalc.com/DrugStats/Drugs/Nabumetone.
- ↑ "Relafen (Nabumetone): Side Effects, Interactions, Warning, Dosage & Uses" (in en). RxList. https://www.rxlist.com/relafen-drug.htm.
- ↑ 11.0 11.1 "FDA Warns that Using a Type of Pain and Fever Medication in Second Half of Pregnancy Could Lead to Complications". U.S. Food and Drug Administration (FDA) (Press release). 15 October 2020. Retrieved 15 October 2020.
 This article incorporates text from this source, which is in the public domain.
This article incorporates text from this source, which is in the public domain.
- ↑ 12.0 12.1 "NSAIDs may cause rare kidney problems in unborn babies". 21 July 2017. https://www.fda.gov/drugs/drug-safety-and-availability/fda-recommends-avoiding-use-nsaids-pregnancy-20-weeks-or-later-because-they-can-result-low-amniotic.
 This article incorporates text from this source, which is in the public domain.
This article incorporates text from this source, which is in the public domain.
 |

