Chemistry:Ruthenium(III) acetate
| |
| Identifiers | |
|---|---|
3D model (JSmol)
|
|
| EC Number |
|
PubChem CID
|
|
| |
| |
| Properties | |
| C12H28BF4O18Ru3 | |
| Molar mass | 850.35 g·mol−1 |
| Appearance | green solid |
| Density | 2.110 g/cm3 |
| Structure | |
| octahedral | |
| Hazards | |
| GHS pictograms |  
|
| GHS Signal word | Danger |
| H318, H410 | |
| P273, P280, P305+351+338, P310, P391, P501 | |
| Related compounds | |
Related compounds
|
Manganese(III) acetate Iron(III) acetate |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
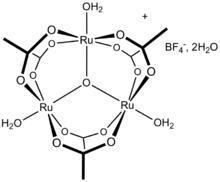
Ruthenium(III) acetate, commonly known as basic ruthenium acetate,[1] describes a family of salts where the cation has the formula [Ru3O(O2CCH3)6(OH2)3]+. A representative derivative is the dihydrate of the tetrafluoroborate salt [Ru3O(O2CCH3)6(OH2)3]BF4(H2O)2, which is the source of the data in the table above.[2] This and related salts are forest green, air-stable solids that are soluble in alcohols.
Basic ruthenium acetate features octahedral Ru(III) centers, a triply bridging oxo ligand, six acetate ligands, and three aquo ligands. The same structure is shared with basic acetates of iron, chromium, iridium, and manganese.[1][2]
Preparation and reactions
It is prepared by heating ruthenium trichloride in acetic acid in the presence of sodium acetate.[3] The basic acetates of ruthenium were reported in the early 1950s but were not properly formulated.[4]
Basic ruthenium acetate reacts with many ligands such as triphenylphosphine and pyridine concomitant with reduction. These derivatives [Ru3O(O2CCH3)6L3]0 are mixed valence compounds.[5]
Related compounds
 [Ru2(OAc)4Cl]n is a coordination polymer with a composition similar to that of ruthenium(III) acetate.
[Ru2(OAc)4Cl]n is a coordination polymer with a composition similar to that of ruthenium(III) acetate.
References
- ↑ 1.0 1.1 Greenwood, Norman N.; Earnshaw, Alan (1997). Chemistry of the Elements (2nd ed.). Butterworth-Heinemann. ISBN 978-0-08-037941-8.
- ↑ 2.0 2.1 O. Almog; A. Bino; D. Garfinkel-Shweky (1993). "The Structure of Oxo-Bridged Trinuclear Ruthenium and Iridium Hexacarboxylates". Inorg. Chim. Acta 213 (1–2): 99. doi:10.1016/S0020-1693(00)83819-0.
- ↑ J. C. Goeltz; S. D. Glover; J. Hauk; C. P. Kubiak (2010). "Ruthenium Complexes". Inorganic Syntheses. 35. 156–160. doi:10.1002/9780470651568.ch8. ISBN 978-0-470-65156-8.
- ↑ Martin, F. S. (1952). "Basic Trinuclear Ruthenium Acetate". Journal of the Chemical Society: 2682–4. doi:10.1039/jr9520002682.
- ↑ Cotton, F. A.; Norman, J. G., Jr. (1972). "Structural Characterization of a Basic trinuclear Ruthenium Acetate". Inorg. Chim. Acta 6: 411–419. doi:10.1016/S0020-1693(00)91829-2.
Acetyl halides and salts of the acetate ion
| |||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| AcOH | He | ||||||||||||||||||
| LiOAc | Be(OAc)2 BeAcOH |
B(OAc)3 | AcOAc ROAc |
NH4OAc | AcOOH | FAc | Ne | ||||||||||||
| NaOAc | Mg(OAc)2 | Al(OAc)3 ALSOL Al(OAc)2OH Al2SO4(OAc)4 |
Si | P | S | ClAc | Ar | ||||||||||||
| KOAc | Ca(OAc)2 | Sc(OAc)3 | Ti(OAc)4 | VO(OAc)3 | Cr(OAc)2 Cr(OAc)3 |
Mn(OAc)2 Mn(OAc)3 |
Fe(OAc)2 Fe(OAc)3 |
Co(OAc)2, Co(OAc)3 |
Ni(OAc)2 | Cu(OAc)2 | Zn(OAc)2 | Ga(OAc)3 | Ge | As(OAc)3 | Se | BrAc | Kr | ||
| RbOAc | Sr(OAc)2 | Y(OAc)3 | Zr(OAc)4 | Nb | Mo(OAc)2 | Tc | Ru(OAc)2 Ru(OAc)3 Ru(OAc)4 |
Rh2(OAc)4 | Pd(OAc)2 | AgOAc | Cd(OAc)2 | In | Sn(OAc)2 Sn(OAc)4 |
Sb(OAc)3 | Te | IAc | Xe | ||
| CsOAc | Ba(OAc)2 | Hf | Ta | W | Re | Os | Ir | Pt(OAc)2 | Au | Hg2(OAc)2, Hg(OAc)2 |
TlOAc Tl(OAc)3 |
Pb(OAc)2 Pb(OAc)4 |
Bi(OAc)3 | Po | At | Rn | |||
| Fr | Ra | Rf | Db | Sg | Bh | Hs | Mt | Ds | Rg | Cn | Nh | Fl | Mc | Lv | Ts | Og | |||
| ↓ | |||||||||||||||||||
| La(OAc)3 | Ce(OAc)x | Pr | Nd | Pm | Sm(OAc)3 | Eu(OAc)3 | Gd(OAc)3 | Tb | Dy(OAc)3 | Ho(OAc)3 | Er | Tm | Yb(OAc)3 | Lu(OAc)3 | |||||
| Ac | Th | Pa | UO2(OAc)2 | Np | Pu | Am | Cm | Bk | Cf | Es | Fm | Md | No | Lr | |||||
 |

