Medicine:Human orthopneumovirus
| Human respiratory syncytial virus infection | |
|---|---|
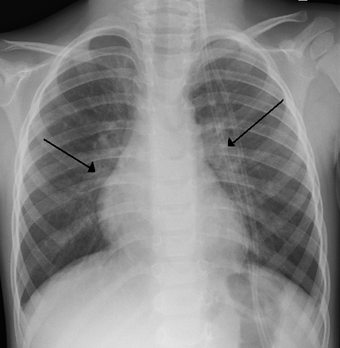 | |
| An x ray of a child with HRSV showing the typical bilateral perihilar fullness | |
| Specialty | Pediatrics |
| Prevention | Hand washing, avoiding close contact with sick people[1] |
Human orthopneumovirus (also known as human respiratory syncytial virus, or HRSV, or just RSV) is a virus that causes respiratory tract infections, with the infected cells of the mucosa fusing together to form a syncytium. It is a major cause of lower respiratory tract infections and hospital visits during infancy and childhood. A prophylactic medication, palivizumab, can be employed to prevent HRSV in preterm (under 35 weeks gestation) infants, infants with certain congenital heart defects (CHD) or bronchopulmonary dysplasia (BPD), and infants with congenital malformations of the airway. Treatment is limited to supportive care, including oxygen therapy and more advanced breathing support with CPAP or nasal high flow oxygen, as required.
In temperate climates there is an annual epidemic during the winter months; in tropical climates, infection is most common during the rainy season.
In the United States, 60% of infants are infected during their first HRSV season,[2] and nearly all children will have been infected with the virus by 2–3 years of age.[2] Of those infected with RSV, 2–3% will develop bronchiolitis, necessitating hospitalization.[3] Natural infection with HRSV induces protective immunity which wanes over time—possibly more so than other respiratory viral infections—and thus people can be infected multiple times. Sometimes an infant can become symptomatically infected more than once, even within a single HRSV season. Severe HRSV infections have increasingly been found among elderly patients. Young adults can be re-infected every five to seven years, with symptoms looking like a sinus infection or a cold (infections can also be asymptomatic).
Signs and symptoms
The incubation time (from infection until symptoms arrive) is 4–5 days. For adults, HRSV produces mainly mild symptoms, often indistinguishable from common colds and minor illnesses. The Centers for Disease Control consider HRSV to be the "most common cause of bronchiolitis (inflammation of the small airways in the lung) and pneumonia in children under 1 year of age in the United States".[4] For some children, RSV can cause bronchiolitis, leading to severe respiratory illness requiring hospitalization and, rarely, causing death. This is more likely to occur in patients that are immunocompromised or infants born prematurely. Other HRSV symptoms common among infants include listlessness, poor or diminished appetite, and a possible fever.
Recurrent wheezing and asthma are more common among individuals who suffered severe HRSV infection during the first few months of life than among controls;[5] whether HRSV infection sets up a process that leads to recurrent wheezing or whether those already predisposed to asthma are more likely to become severely ill with HRSV has yet to be determined.
Symptoms of pneumonia in immuno-compromised patients such as in transplant patients and especially bone marrow transplant patients should be evaluated to rule out HRSV infection. This can be done by means of polymerase chain reaction (PCR) testing for HRSV nucleic acids in peripheral blood samples if all other infectious processes have been ruled out or if it is highly suspicious for RSV such as a recent exposure to a known source of HRSV infection. The incubation period is 2–8 days but is usually 4–6 days.
Complications
- bronchiolitis or pneumonia
- asthma
- recurring infections
- acute otitis media
Cause
Virology
| Human orthopneumovirus | |
|---|---|
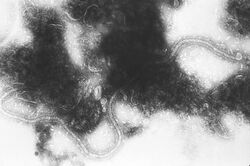
| |
| Transmission electron micrograph of RSV | |
| Virus classification | |
| (unranked): | Virus |
| Realm: | Riboviria |
| Kingdom: | Orthornavirae |
| Phylum: | Negarnaviricota |
| Class: | Monjiviricetes |
| Order: | Mononegavirales |
| Family: | Pneumoviridae |
| Genus: | Orthopneumovirus |
| Species: | Human orthopneumovirus
|
| Synonyms[6] | |
| |
Structure
Human respiratory syncytial virus is a medium-sized (120–200 nm) enveloped virus that contains a linear negative-sense RNA genome (must be converted to a positive RNA prior to translation). The former contains virally encoded F, G, and SH lipoproteins. The F and G lipoproteins are the only two that target the cell membrane and are highly conserved among RSV isolates. HRSV is divided into two antigenic subgroups, A and B, on the basis of the reactivity of the virus with monoclonal antibodies against the attachment (G) and fusion (F)[7] glycoproteins. Subtype B is characterized as the asymptomatic strains of the virus that the majority of the population experiences. The more severe clinical illnesses involve subtype A strains, which tend to predominate in most outbreaks.
Four of the viral genes code for intracellular proteins that are involved in genome transcription, replication, and particle budding, namely N (nucleoprotein), P (phosphoprotein), M (matrix protein), and L (“large” protein, containing the RNA polymerase catalytic motifs). The RSV genomic RNA forms a helical ribonucleoprotein (RNP) complex with the N protein, termed nucleocapsid, which is used as template for RNA synthesis by the viral polymerase complex. The three-dimensional crystal structure of a decameric, annular ribonucleoprotein complex of the RSV nucleoprotein (N) bound to RNA has been determined at 3.3 Å resolution. This complex mimics one turn of the viral helical nucleocapsid complex. Its crystal structure was combined with electron microscopy data to provide a detailed model for the RSV nucleocapsid.[8]
Genome
The genome is approximately 15,000 nucleotides in length and is composed of a single strand of RNA with negative polarity. It has 10 genes encoding 11 proteins.[9]
RSV, and the closely related Pneumonia Virus of Mice, are unique in the Paramyxoviridae family of RNA viruses for having two separate genes coding for nonstructural proteins (NS1, NS2) that suppress the host's innate immunity, mediated primarily by type I interferon (IFN).[10][11]
To date, 10 HRSV-A genotypes have been designated, GA1 to GA7, SAA1, NA1, and NA2. The HRSV-B genotypes include GB1 to GB4, SAB1 to SAB3, and BA1 to BA6.[9]
The genome of HRSV was completely sequenced in 1997.
Evolution
Bayesian estimates of the mutation rates in the subtype A genomes give a mutation rate of 6.47×10−4 (credible interval: 5.56×10−4 – 7.38×10−4) substitutions/site/year.[12] This is similar to other RNA viruses. The population size has remained constant over the last 70 years and the G protein appears to be the main site of diversifying selection. The most recent common ancestor evolved ~1943 (credible interval: 1923–1954). The HRSV-B evolutionary rate (1.95×10−3 nucleotide substitutions/site/year) is similar to that previously estimated for HRSV-A (1.83×10−3 nucleotide substitutions/site/year). However, natural HRSV-B isolates appear to accommodate more drastic changes in their attachment G proteins. The most recent common ancestor of the currently circulating subgroup B strains was estimated to date back to around the year 1949. The divergence between the two major subgroups was calculated to have occurred approximately 350 years ago (mid-17th century).
Taxonomy
HRSV is a negative-sense, single-stranded RNA virus of the family Pneumoviridae. Its name comes from the fact that F proteins on the surface of the virus cause the cell membranes on nearby cells to merge, forming syncytia.
Transmission
HRSV spreads easily by direct contact, and can remain viable for a half an hour or more on hands or for up to 5 hours on countertops.[13] Childcare facilities allow for rapid child-to-child transmission in a short period of time.[14]
The HRSV is virtually the same as chimpanzee coryza virus and can be transmitted from apes to humans, although transmission from humans to apes is more common.[15] The virus has also been recovered from cattle, goats and sheep, but these are not regarded as major vectors of transmission and there is no animal reservoir of the virus.
HRSV infection can last 2–8 days, but symptoms may persist for up to three weeks.
Diagnosis
Human respiratory syncytial virus may be suspected based on the time of year of the infection; prevalence usually coincides with the winter flu season.
- Physical exam: listening with a stethoscope for wheezing and other abnormal sounds in the chest
- Chest X-rays to check for typical bilateral perihilar fullness of bronchiolitis induced by the virus
- Skin monitoring to check for hypoxemia, a lower than usual level of oxygen in the bloodstream
- Blood tests to check white cell counts or to look for the presence of viruses, bacteria or other organisms
- Lab test of respiratory secretions
Several different types of laboratory tests are commercially available for diagnosis of RSV infection. Rapid diagnostic assays performed on respiratory specimens are available commercially. Most clinical laboratories currently use antigen detection tests. Compared with culture, the sensitivity of antigen detection tests generally ranges from 80% to 90%. Antigen detection tests and culture are generally reliable in young children but less useful in older children and adults.
Sensitivity of virus isolation from respiratory secretions in cell culture varies among laboratories. RT-PCR assays are now commercially available. The sensitivity of these assays is equal to or exceeds the sensitivity of virus isolation and antigen detection methods. Highly sensitive RT-PCR assays should be considered when testing adults, because they may have low viral loads in their respiratory specimens.
Serologic tests are less frequently used for diagnosis. Although useful for research, a diagnosis using a collection of paired acute and convalescent sera to demonstrate a significant rise in antibody titre to HRSV can not be made in time to guide care of the patient. On top of that, the antibody level does not always correlate with the acuteness or activity level of the infection.
RSV infection can be confirmed using tests for antigens or antibodies, or viral RNA by reverse transcription PCR. Quantification of viral load can be determined by various assay tests.
Prevention
As the virus is ubiquitous in all parts of the world, avoidance of infection is not possible.[citation needed]
However, palivizumab (brand name Synagis manufactured by MedImmune), a moderately effective prophylactic drug, is available for infants at high risk. Palivizumab is a monoclonal antibody directed against RSV surface fusion protein. It is given by monthly injections, which are begun just prior to the RSV season and are usually continued for five months. HRSV prophylaxis is indicated for infants that are premature or have either cardiac or lung disease, but the cost of prevention limits use in many parts of the world.[16]
Treatment
To date, treatment has been limited to supportive measures. Adrenaline, bronchodilators, steroids, antibiotics, and ribavirin confer "no real benefit".[17][18]
Studies of nebulized hypertonic saline have shown that the "use of nebulized 3% HS is a safe, inexpensive, and effective treatment for infants hospitalized with moderately severe viral bronchiolitis" where "respiratory syncytial virus (RSV) accounts for the majority of viral bronchiolitis cases".[19][20] One study noted a 26% reduction in length of stay: 2.6 ± 1.9 days, compared with 3.5 ± 2.9 days in the normal-saline treated group (p=0.05).[19]
Supportive care includes fluids and supplemental oxygen until the illness runs its course. Salbutamol may be used in an attempt to relieve any bronchospasm if present. Increased airflow, humidified and delivered via nasal cannula, may be supplied in order to reduce the effort required for respiration.
Presatovir, an experimental antiviral drug, has shown promising results in clinical trials but has not yet been approved for medical use.[21]
Research
As of 2017 there was no respiratory syncytial virus vaccine despite many years of preclinical and clinical work.[22] A formalin-inactivated candidate developed in the 1960s surprisingly increased infections, which came to be known as "immunopotentiation" or vaccine-enhanced disease.[23][24]
As of 2017 several companies including Novavax, GlaxoSmithKline and Bavarian Nordic were working on RSV vaccines.[25][26][27]
See also
- Robert M. Chanock, discoverer of the human respiratory syncytial virus
- Metapneumovirus, another genus of closely related viruses
- Orthopneumovirus, the genus of related syncytial viruses
References
- ↑ https://www.nhs.uk/conditions/bronchiolitis/prevention/
- ↑ 2.0 2.1 Glezen, WP; Taber, LH; Frank, AL; Kasel, JA (1986). "Risk of primary infection and reinfection with respiratory syncytial virus". American Journal of Diseases of Children 140 (6): 543–6. doi:10.1001/archpedi.1986.02140200053026. PMID 3706232.
- ↑ Hall, Caroline Breese; Weinberg, Geoffrey A.; Iwane, Marika K.; Blumkin, Aaron K.; Edwards, Kathryn M.; Staat, Mary A.; Auinger, Peggy; Griffin, Marie R. et al. (2009). "The Burden of Respiratory Syncytial Virus Infection in Young Children". New England Journal of Medicine 360 (6): 588–98. doi:10.1056/NEJMoa0804877. PMID 19196675.
- ↑ "Respiratory Syncytial Virus". Center for Disease Control, Respiratory and Enteric Viruses Branch. October 17, 2008. https://www.cdc.gov/rsv/index.html. Retrieved October 2, 2009.
- ↑ Wu, P.; Dupont, W. D.; Griffin, M. R.; Carroll, K. N.; Mitchel, E. F.; Gebretsadik, T.; Hartert, T. V. (2008). "Evidence of a Causal Role of Winter Virus Infection during Infancy in Early Childhood Asthma". American Journal of Respiratory and Critical Care Medicine 178 (11): 1123–9. doi:10.1164/rccm.200804-579OC. PMID 18776151.
- ↑ "ICTV Taxonomy history: Human orthopneumovirus" (in en). https://talk.ictvonline.org/taxonomy/p/taxonomy-history?taxnode_id=20181651. Retrieved 27 December 2018.
- ↑ "A highly stable prefusion RSV F vaccine derived from structural analysis of the fusion mechanism". Nature Communications 6: 8143. 2015. doi:10.1038/ncomms9143. PMID 26333350. Bibcode: 2015NatCo...6.8143K.
- ↑ Tawar, RG; Duquerroy, S; Vonrhein, C; Varela, PF; Damier-Piolle, L; Castagné, N; MacLellan, K; Bedouelle, Hugues et al. (November 2009). "Crystal structure of a nucleocapsid-like nucleoprotein-RNA complex of respiratory syncytial virus". Science 326 (5957): 1279–83. doi:10.1126/science.1177634. PMID 19965480. Bibcode: 2009Sci...326.1279T.
- ↑ 9.0 9.1 Lee, Wan-Ji; Kim, You-jin; Kim, Dae-Won; Lee, Han Saem; Lee, Ho Yeon; Kim, Kisoon (2012-12-15). "Complete Genome Sequence of Human Respiratory Syncytial Virus Genotype A with a 72-Nucleotide Duplication in the Attachment Protein G Gene". Journal of Virology 86 (24): 13810–13811. doi:10.1128/JVI.02571-12. PMID 23166231.
- ↑ Barik S (2013) Respiratory syncytial virus mechanisms to interfere with type 1 interferons. Curr Top Microbiol Immunol. 372:173-91. doi: 10.1007/978-3-642-38919-1_9. PMID 24362690.
- ↑ Ribaudo M, Barik S (2017) The nonstructural proteins of Pneumoviruses are remarkably distinct in substrate diversity and specificity. Virol J. ;14(1):215. doi: 10.1186/s12985-017-0881-7, PMID 29110727.
- ↑ Leung, Frederick C. C, ed (2012). "Genetic Variability among Complete Human Respiratory Syncytial Virus Subgroup A Genomes: Bridging Molecular Evolutionary Dynamics and Epidemiology". PLOS One 7 (12): e51439. doi:10.1371/journal.pone.0051439. PMID 23236501. Bibcode: 2012PLoSO...751439T.
- ↑ "Respiratory syncytial virus (RSV)". A.D.A.M. Medical Encyclopedia, PubMed Health. National Center for Biotechnology Information, U.S. National Library of Medicine. January 2011. https://www.ncbi.nlm.nih.gov/pubmedhealth/PMH0002531/.
- ↑ Chu, H. Y.; Kuypers, J.; Renaud, C.; Wald, A.; Martin, E.; Fairchok, M.; Magaret, A.; Sarancino, M. et al. (2013). "Molecular epidemiology of respiratory syncytial virus transmission in childcare". Journal of Clinical Virology 57 (4): 343–350. doi:10.1016/j.jcv.2013.04.011. PMID 23684816.
- ↑ http://c.ymcdn.com/sites/www.aazv.org/resource/resmgr/IDM/IDM_Respiratory_Syncytial_Vi.pdf American Association of Zoo Veterinarians Infectious Disease Committee Manual 2013, CHIMPANZEE CORYZA/RESPIRATORY SYNCYTIAL VIRUS (RSV), Allison Wack, December 26, 2010, updated March 19, 2013.
- ↑ Andabaka, Tea; Nickerson, Jason W; Rojas-Reyes, Maria Ximena; Rueda, Juan David; Bacic Vrca, Vesna; Barsic, Bruno (30 April 2013). "Monoclonal antibody for reducing the risk of respiratory syncytial virus infection in children". Cochrane Database of Systematic Reviews (4): CD006602. doi:10.1002/14651858.CD006602.pub4. PMID 23633336.
- ↑ Bourke TW, Shields MD. Bronchiolitis. BMJ Clinical Evidence. 2011;04:308
- ↑ Handforth, J.; Sharland, M; Friedland, JS (2004). "Prevention of respiratory syncytial virus infection in infants". BMJ 328 (7447): 1026–7. doi:10.1136/bmj.328.7447.1026. PMID 15117767.
- ↑ 19.0 19.1 Kuzik, BA; Al Qadhi, SA; Kent, S; Flavin, MP; Hopman, W; Hotte, S; Gander, S (2007). "Nebulized hypertonic saline in the treatment of viral bronchiolitis in infants". The Journal of Pediatrics 151 (3): 266–70, 270.e1. doi:10.1016/j.jpeds.2007.04.010. PMID 17719935.
- ↑ Mandelberg, A.; Tal, G; Witzling, M; Someck, E; Houri, S; Balin, A; Priel, IE (2003). "Nebulized 3% Hypertonic Saline Solution Treatment in Hospitalized Infants With Viral Bronchiolitis". Chest 123 (2): 481–7. doi:10.1378/chest.123.2.481. PMID 12576370.
- ↑ Beigel JH, Nam HH, Adams PL, Krafft A, Ince WL, El-Kamary SS, Sims AC. Advances in respiratory virus therapeutics - A meeting report from the 6th isirv Antiviral Group conference. Antiviral Res. 2019 Jul;167:45-67. PMID 30974127 doi:10.1016/j.antiviral.2019.04.006
- ↑ Schmidt, ME; Varga, SM (March 2017). "Modulation of the host immune response by respiratory syncytial virus proteins.". Journal of Microbiology (Seoul, Korea) 55 (3): 161–171. doi:10.1007/s12275-017-7045-8. PMID 28243940.
- ↑ Melero, JA (October 2016). "Influence of antigen conformation and mode of presentation on the antibody and protective responses against human respiratory syncytial virus: relevance for vaccine development.". Expert Review of Vaccines 15 (10): 1319–25. doi:10.1080/14760584.2016.1175941. PMID 27055009.
- ↑ Schmidt, Megan E.; Knudson, Cory J.; Hartwig, Stacey M.; Pewe, Lecia L.; Meyerholz, David K.; Langlois, Ryan A.; Harty, John T.; Varga, Steven M. (January 2018). "Memory CD8 T cells mediate severe immunopathology following respiratory syncytial virus infection". PLOS Pathogens 14 (1): e1006810. doi:10.1371/journal.ppat.1006810. ISSN 1553-7374. PMID 29293660.
- ↑ "GSK's RSV vaccine product development overview". WHO. http://www.who.int/immunization/research/meetings_workshops/GSK_RSV_vaccdev_status_Dieussaert.pdf. Retrieved 2017-07-04.
- ↑ Esposito, S; Pietro, GD (October 2016). "Respiratory syncytial virus vaccines: an update on those in the immediate pipeline.". Future Microbiology 11 (11): 1479–1490. doi:10.2217/fmb-2016-0106. PMID 27750448.
- ↑ "Clinical Stage Pipeline – Novavax". http://novavax.com/page/11/clinical-stage-pipeline.
External links
| Classification | |
|---|---|
| External resources |
- discoverrsv.com (registered to Novavax, manufacturer of RSV vaccines Prepare™ and Resolve™ for maternal/infant and elderly RSV immunizations)
- PreemieCare information and support on premature infants including in-depth resources on RSV and our comprehensive NICU Glossary.
- Synagis (registered to MedImmune, manufacturer of Synagis)
- Virazole (registered to Valeant Pharmaceuticals, manufacturer of Virazole)
- The Family Doctor
- RSV in Infants: Information includes symptoms, treatment, and prevention
- Biotrin providers of RSV kits
- Control of Communicable Diseases in Man. American Public Health Association.
- [1]
Wikidata ☰ Q1052913 entry

