Medicine:Viral hepatitis
| Viral hepatitis | |
|---|---|
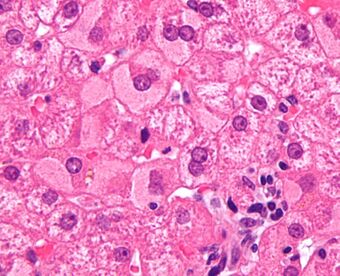 | |
| Micrograph showing ground glass hepatocytes, which are seen in chronic hepatitis B infections (a type of viral hepatitis), and represent accumulations of viral antigen in the endoplasmic reticulum. H&E stain. |
Viral hepatitis is liver inflammation due to a viral infection.[1][2] It may present in acute form as a recent infection with relatively rapid onset, or in chronic form.
The most common causes of viral hepatitis are the five unrelated hepatotropic viruses hepatitis A, B, C, D, and E. Other viruses can also cause liver inflammation, including cytomegalovirus, Epstein-Barr virus, and yellow fever. There also have been scores of recorded cases of viral hepatitis caused by herpes simplex virus.[3]
Mode Of Transmission
Viral hepatitis is either transmitted through contaminated food or water (A, E) or via blood and body fluids (B, C). The viruses which get transmitted through water and food are mostly self-limited resulting in acute illness with full resolution. The blood borne viruses (B, C) can cause both acute and chronic liver disease and can be transmitted from mother to child during birth, through contact with body fluids during sex, unsafe injections and through unscreened blood transfusions.[4]
The most common types of hepatitis can be prevented or treated.[5] Hepatitis A and hepatitis B can be prevented by vaccination. Effective treatments for hepatitis C are available but costly.[5]
In 2013, about 1.5 million people died from viral hepatitis, most commonly due to hepatitis B and C.[5] East Asia, in particular Mongolia, is the region most affected.[5]
Hepatitis viruses
The most common cause of hepatitis is viral. Although the effects of various viruses are all classified under the disease hepatitis, these viruses are not all related.[citation needed]
| Hepatitis A virus (HAV) | Hepatitis B virus (HBV) | Hepatitis C virus (HCV) | Hepatitis D virus (HDV) | Hepatitis E virus (HEV) | Hepatitis G virus (HGV) | |
|---|---|---|---|---|---|---|
| Viral species | Hepatovirus A | Hepatitis B virus | Hepacivirus C | Hepatitis delta virus | Orthohepevirus A | Also known as Human Pegvirus (HPgV) and as GB virus C (GBV-C) |
| Viral family | Picornaviridae | Hepadnaviridae | Flaviviridae | Incertae sedis | Hepeviridae | Flaviviridae |
| Genome | (+)ssRNA | dsDNA-RT | (+)ssRNA | (−)ssRNA | (+)ssRNA | (+)ssRNA |
| Antigens | HBsAg, HBeAg | Core antigen | Delta antigen | |||
| Transmission | Enteral | Parenteral | Parenteral | Parenteral | Enteral | Parenteral |
| Incubation period | 20–40 days | 45–160 days | 15–150 days | 30–60 days | 15–60 days | 14-20 days |
| Severity/Chronicity[6] | Mild; acute | Occasionally severe; 5–10% chronic | Subclinical; 70% chronic | Exacerbates symptoms of HBV; chronic with HBV | Mild in normal patients; severe in pregnant women; acute | |
| Vaccine | 2 injections; at least 20 years of protection[7] | 3 injections; lifetime protection | None available | None available, but not considered necessary; Hep B vaccine provides protection[8] | Investigational (approved in China) |
Viral hepatitis types
Hepatitis A
Hepatitis A or infectious jaundice is caused by hepatitis A virus (HAV), a picornavirus transmitted by the fecal-oral route often associated with ingestion of contaminated food. It causes an acute form of hepatitis and does not have a chronic stage. The patient's immune system makes antibodies against HAV that confer immunity against future infection. People with hepatitis A are advised to rest, stay hydrated and avoid alcohol. A vaccine is available that will prevent HAV infection for up to 10 years. Hepatitis A can be spread through personal contact, consumption of raw sea food, or drinking contaminated water. This occurs primarily in third world countries. Strict personal hygiene and the avoidance of raw and unpeeled foods can help prevent an infection. Infected people excrete HAV with their feces two weeks before and one week after the appearance of jaundice. The time between the infection and the start of the illness averages 28 days (ranging from 15 to 50 days),[9] and most recover fully within 2 months, although approximately 15% of sufferers may experience continuous or relapsing symptoms from six months to a year following initial diagnosis.[10]
| Marker | Detection Time | Description | Significance |
|---|---|---|---|
| Faecal HAV | 2–4 weeks or 28 days | – | Early detection |
| Ig M anti HAV | 4–12 weeks | Enzyme immunoassay for antibodies | During acute Illness |
| Ig G anti HAV | 5 weeks–persistent | Enzyme immunoassay for antibodies | Old infection or reinfection |
Hepatitis B
Hepatitis B is caused by the hepatitis B virus, a hepadnavirus that can cause both acute and chronic hepatitis. Chronic hepatitis develops in the 15% of adults who are unable to eliminate the virus after an initial infection. Identified methods of transmission include contact with blood, blood transfusion (now rare), unsanitary tattoos, sex (through sexual intercourse or contact with bodily fluids), or mother-to-child by breast feeding;[citation needed] there is minimal evidence of transplacental crossing. However, in about half of cases the source of infection cannot be determined. Blood contact can occur by sharing syringes in intravenous drug use, shaving accessories such as razor blades, or touching wounds on infected persons. Needle-exchange programmes have been created in many countries as a form of prevention.[citation needed]
Patients with chronic hepatitis B have antibodies against the virus, but not enough to clear the infected liver cells. The continued production of virus and countervailing antibodies is a likely cause of the immune complex disease seen in these patients. A vaccine is available to prevent infection for life. Hepatitis B infections result in 500,000 to 1,200,000 deaths per year worldwide due to the complications of chronic hepatitis, cirrhosis, and hepatocellular carcinoma. Hepatitis B is endemic in a number of (mainly South-East Asian) countries, making cirrhosis and hepatocellular carcinoma big killers. There are eight treatment options approved by the U.S. Food and Drug Administration (FDA) available for persons with a chronic hepatitis B infection: alpha-interferon, pegylated interferon, adefovir, entecavir, telbivudine, lamivudine, tenofovir disoproxil and tenofovir alafenamide with a 65% rate of sustained response.[citation needed]
Hepatitis C
Hepatitis C (originally "non-A non-B hepatitis") is caused by hepatitis C virus (HCV), an RNA virus of the family Flaviviridae. HCV can be transmitted through contact with blood (including through sexual contact if the two parties' blood is mixed) and can also cross the placenta. Hepatitis C usually leads to chronic hepatitis, culminating in cirrhosis in some people. It usually remains asymptomatic for decades. Patients with hepatitis C are susceptible to severe hepatitis if they contract either hepatitis A or B, so all persons with hepatitis C should be immunized against hepatitis A and hepatitis B if they are not already immune, and avoid alcohol. HCV can lead to the development of hepatocellular carcinoma, however, only a minority of HCV-infected individuals develop cancer (1-4% annually), suggesting a complex interplay between viral gene expression and host and environmental factors to promote carcinogenesis. The risk is increased two-fold with active HBV coinfection and a 21% increase in mortality compared to those with latent HBV and HCV.[12] HCV viral levels can be reduced to undetectable levels by a combination of interferon and the antiviral drug ribavirin. The genotype of the virus is the primary determinant of the rate of response to this treatment regimen, with genotype 1 being the most resistant.[citation needed]
Hepatitis C is the most common chronic blood-borne infection in the United States.[13]
| Marker | Detection Time | Description | Significance | Note |
|---|---|---|---|---|
| HCV-RNA | 1–3 weeks or 21 days | PCR | Demonstrates presence or absence of virus | Results may be intermittent during course of infection. Negative result is not indicative of absence. |
| anti-HCV | 5–6 weeks | Enzyme Immunoassay for antibodies | Demonstrates past or present infection | High false positive in those with autoimmune disorders and populations with low virus prevalence. |
| ALT | 5–6 weeks | – | Peak in ALT coincides with peak in anti-HCV | Fluctuating ALT levels is an indication of active liver disease. |
Hepatitis D
Hepatitis D is caused by the hepatitis D virus (HDV), or hepatitis delta virus; it belongs to the genus Deltavirus. It is similar to a satellite virus as it can only propagate in the presence of the hepatitis B virus, depending on the helper function of HBV for its replication and expression. It has no independent life cycle, but can survive and replicate as long as HBV infection persists in the host body. It can only cause infection when encapsulated by hepatitis B virus surface antigens. The vaccine for hepatitis B protects against hepatitis D virus because of the latter's dependence on the presence of hepatitis B virus for it to replicate.[15][8]
Hepatitis E
Hepatitis E is caused by the Hepatitis E virus (HEV), from the family Hepeviridae; it produces symptoms similar to hepatitis A, although it can take a fulminant course in some patients, particularly pregnant women (mortality rate about 20%); chronic infections may occur in immune-compromised patients. It is more prevalent in the Indian subcontinent. The virus is feco-orally transmitted and usually is self-limited.[citation needed]
Hepatitis F virus
Hepatitis F virus (HFV) is a hypothetical virus linked to certain cases of hepatitis. Several hepatitis F virus candidates emerged in the 1990s, but none of these reports have been substantiated.[citation needed]
GB virus C
The GB virus C is another potential viral cause of hepatitis that is probably spread by blood and sexual contact.[16] It was initially identified as Hepatitis G virus.[17] There is very little evidence that this virus causes hepatitis, as it does not appear to replicate primarily in the liver.[18] It is now classified as GB virus C.[19]
Relationship between hepatitis C virus and liver cancer
Hepatitis C virus (HCV) are causing acute and chronic infections that is a major cause of hepatocellular carcinoma (HCC), advanced hepatic fibrosis and cirrhosis.[citation needed]
A major cause of death in HCC patients with chronic HCV infection. The pathogenesis of HCC associated with HCV, that virus play direct and indirect roles.[20]
A major risk for the development of HCC is persistent infection with HCV and the highest risk for HCC development is associated with co-infection of HBV with HDV, HCV or HIV.[21]
The risk factors lead to development of HCC in chronic HCV is synchronous liver diseases, viral genotype, lifestyle factors and presence of obesity and diabetes mellitus. The lifestyle factors such as smoking, alcohol use and coffee drinking accelerated progression to HCC in HCV.[citation needed]
The purpose of HCV treatment is to eliminate the infection, reduce the transmission to other people and decrease the risk of HCC development.[citation needed]
Other viruses
The virus first known to cause hepatitis was the yellow fever virus, a mosquito-borne flavivirus. Other viruses than can cause hepatitis include:
- Adenoviruses
- Arenaviruses: Guanarito virus, Junín virus, Lassa fever virus, Lujo virus, Machupo virus, and Sabiá virus[22]
- Bunyaviruses: Crimean-Congo hemorrhagic fever virus, Dobrava virus, Hantaan virus, Puumala virus, Rift Valley fever virus, and Seoul virus
- Coronavirus: severe acute respiratory syndrome virus[23]
- Erythrovirus: Parvovirus B19[24]
- Filoviruses: Ebola virus and Marburg virus
- Flaviviruses: dengue, Kyasanur Forest disease virus, Omsk hemorrhagic fever virus, and yellow fever virus
- Herpesviruses: cytomegalovirus,[25]Epstein–Barr virus,[26] varicella-zoster virus,[27] human herpesvirus 6, human herpesvirus 7, and human herpesvirus 8[28]
- Orthomyxoviruses: influenza[29]
- Picornaviruses: echovirus
- Reovirus: Colorado tick fever virus, reovirus 3
KIs-V is a virus isolated in 2011 from four patients with raised serum alanine transferases without other known cause; a causal role is suspected.[30]
References
- ↑ National Library of Medicine » Medical Subject Headings »Virus Diseases (C02) » Hepatitis, Viral, Human (C02.440) » Scope Note. https://www.nlm.nih.gov/cgi/mesh/2011/MB_cgi?mode=&index=6261&view=expanded.
- ↑ "Hepatitis | MedlinePlus" (in en). https://medlineplus.gov/hepatitis.html.
- ↑ Herpes Simplex Virus Hepatitis: Case Report and Review
- ↑ "Hepatitis B". https://www.who.int/news-room/fact-sheets/detail/hepatitis-b."Hepatitis C". https://www.who.int/news-room/fact-sheets/detail/hepatitis-c.
- ↑ 5.0 5.1 5.2 5.3 Stanaway, Jeffrey D; Flaxman, Abraham D; Naghavi, Mohsen; Fitzmaurice, Christina; Vos, Theo; Abubakar, Ibrahim; Abu-Raddad, Laith J; Assadi, Reza et al. (July 2016). "The global burden of viral hepatitis from 1990 to 2013: findings from the Global Burden of Disease Study 2013". The Lancet 388 (10049): 1081–8. doi:10.1016/S0140-6736(16)30579-7. PMID 27394647.
- ↑ Kuo, Infectious Causes of Jaundice, ATSU School of Osteopathic Medicine Arizona, June 2012
- ↑ "Hepatitis a Q&As for Health Professionals | CDC". 9 April 2021. https://www.cdc.gov/hepatitis/hav/havfaq.htm#B5.
- ↑ 8.0 8.1 Desk Encyclopedia of Human and Medical Virology. Boston: Academic Press. 2009. p. 121. ISBN 978-0-12-375147-8. https://archive.org/details/deskencyclopedia00mahy.
- ↑ "CDC Hepatitis A FAQ". https://www.cdc.gov/ncidod/diseases/hepatitis/a/faqa.htm#general.
- ↑ "CDC Hepatitis A Fact Sheet". https://www.cdc.gov/ncidod/diseases/hepatitis/a/fact.htm.
- ↑ "Acute Viral Hepatitis : Introduction Harrison's Principle of Internal Medicine, 17 Edition". http://www.accessmedicine.com.
- ↑ Ringehan, Marc; McKeating, Jane A.; Protzer, Ulrike (2017-10-19). "Viral hepatitis and liver cancer". Philosophical Transactions of the Royal Society B: Biological Sciences 372 (1732): 20160274. doi:10.1098/rstb.2016.0274. ISSN 0962-8436. PMID 28893941.
- ↑ "CDC DVH—Hepatitis C Information For the Health Professional". https://www.cdc.gov/hepatitis/HCV/index.htm.
- ↑ "WHO | Hepatitis C". Who.int. 2010-12-08. https://www.who.int/csr/disease/hepatitis/whocdscsrlyo2003/en/index3.html#diagnostic.
- ↑ U.S. National Library of Medicine "Delta Agent (hepatitis D)"
- ↑ "Detection of the hepatitis G virus genome among injecting drug users, homosexual and bisexual men, and blood donors". J. Infect. Dis. 174 (6): 1320–3. 1996. doi:10.1093/infdis/174.6.1320. PMID 8940225.
- ↑ "Molecular cloning and disease association of hepatitis G virus: a transfusion-transmissible agent". Science 271 (5248): 505–8. 1996. doi:10.1126/science.271.5248.505. PMID 8560265. Bibcode: 1996Sci...271..505L.
- ↑ "Quantitation of hepatitis G and C viruses in the liver: evidence that hepatitis G virus is not hepatotropic". Hepatology 27 (3): 877–80. 1998. doi:10.1002/hep.510270335. PMID 9500722.
- ↑ "00.026. Flaviviridae". ICTVdB Index of Viruses. http://phene.cpmc.columbia.edu/Ictv/fs_flavi.htm#Genus0.
- ↑ de Oliveria Andrade, Luis Jesuino; D'Oliveira, Argemiro; Melo, Rosangela Carvalho; De Souza, Emmanuel Conrado; Costa Silva, Carolina Alves; Paraná, Raymundo (2009). "Association Between Hepatitis C and Hepatocellular Carcinoma". Journal of Global Infectious Diseases 1 (1): 33–37. doi:10.4103/0974-777X.52979. ISSN 0974-777X. PMID 20300384.
- ↑ Ringehan, Marc; McKeating, Jane A.; Protzer, Ulrike (2017-10-19). "Viral hepatitis and liver cancer". Philosophical Transactions of the Royal Society B: Biological Sciences 372 (1732). doi:10.1098/rstb.2016.0274. ISSN 0962-8436. PMID 28893941.
- ↑ "[The other types of viral hepatitis]" (in fr). Rev Prat 40 (18): 1656–9. 1990. PMID 2164704.
- ↑ "SARS-associated viral hepatitis caused by a novel coronavirus: report of three cases". Hepatology 39 (2): 302–10. February 2004. doi:10.1002/hep.20111. PMID 14767982.
- ↑ Naides SJ (May 1998). "Rheumatic manifestations of parvovirus B19 infection". Rheum. Dis. Clin. North Am. 24 (2): 375–401. doi:10.1016/S0889-857X(05)70014-4. PMID 9606764.
- ↑ Xiong W (November 2010). "[Clinical efficacy of treating infant cytomegalovirus hepatitis with ganciclovir and impact on cytokines]" (in zh). Xi Bao Yu Fen Zi Mian Yi Xue Za Zhi 26 (11): 1130–2. PMID 21322282.
- ↑ Okano M, Gross TG; Gross (November 2011). "Acute or Chronic Life-Threatening Diseases Associated With Epstein-Barr Virus Infection". Am. J. Med. Sci. 343 (6): 483–9. doi:10.1097/MAJ.0b013e318236e02d. PMID 22104426.
- ↑ Anderson, D.R.; Schwartz, J.; Hunter, N.J.; Cottrill, C.; Bissaccia, E.; Klainer, A.S. (1994). "Varicella Hepatitis: A Fatal Case in a Previously Healthy, Immunocompetent Adult". Archives of Internal Medicine 154 (18): 2101–2106. doi:10.1001/archinte.1994.00420180111013. PMID 8092915.
- ↑ Gallegos-Orozco JF, Rakela-Brödner J; Rakela-Brödner (October 2010). "Hepatitis viruses: not always what it seems to be". Rev Med Chil 138 (10): 1302–11. doi:10.4067/S0034-98872010001100016. PMID 21279280.
- ↑ "Liver involvement during influenza infection: perspective on the 2009 influenza pandemic". Influenza and Other Respiratory Viruses 6 (3): e2–5. September 2011. doi:10.1111/j.1750-2659.2011.00287.x. PMID 21951624.
- ↑ "Novel DNA sequence isolated from blood donors with high transaminase levels". Hepatology Research 41 (10): 971–81. October 2011. doi:10.1111/j.1872-034X.2011.00848.x. PMID 21718400.
External links
| Classification |
|---|

